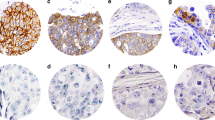
Abstract
Although the prognostic and predictive significance of human epidermal growth factor receptor 2 (HER2) in invasive breast cancer is well established, its role in ductal carcinoma in situ (DCIS) remains unclear. Reports on combined evaluation of both HER2 protein expression and HER2 amplification status in pure DCIS and DCIS adjacent to invasive ductal carcinoma (i.e., admixed DCIS) are scarce. In this study, immunohistochemistry and fluorescence in situ hybridization (FISH) were used to assess HER2 status in 72 cases of pure DCIS, 73 cases of DCIS admixed with invasive ductal carcinoma (IDC), and 60 cases of pure IDC. HER2 copy number-based amplification was present in 49% of pure DCIS, 16% of admixed DCIS, 18% of admixed IDC, and 8% of pure IDC. Amplified pure DCIS with clusters of HER2 signals showed a significantly lower HER2 copy number than amplified admixed DCIS with clusters. Whereas pure DCIS and admixed DCIS presented significant differences, the in situ and invasive component of admixed tumors showed striking similarities regarding mean HER2 and chromosome 17 centromere (CEP17) copy number, grade, and estrogen and progesterone receptor expression. The discrepant prevalence of HER2 amplification among breast cancer subgroups indirectly suggests that HER2 may not play a crucial role in the transition of in situ to invasive breast cancer. The similarities in HER2 amplification status between the in situ and invasive component of admixed tumors hint at a common biological pathway for both components. Our data support the theory that pure DCIS, pure IDC, and admixed lesions have a common progenitor, but can progress as separate lineages.


Similar content being viewed by others
Abbreviations
- CEP:
-
Chromosome enumeration probe
- DCIS:
-
Ductal carcinoma in situ
- ER:
-
Estrogen receptor
- FISH:
-
Fluorescence in situ hybridization
- HER2:
-
Human epidermal growth factor receptor 2
- IDC:
-
Invasive ductal carcinoma
- IHC:
-
Immunohistochemistry
- PR:
-
Progesterone receptor
References
Allred DG, Clark GM, Molina R, Tandon AK, Schnitt SJ, Gilchrist KW, Osborne CK, Tormey DC, McGuire WL (1992) Overexpression of HER-2/neu and its relationship with other prognostic factors change during the progression of in situ to invasive breast cancer. Hum Pathol 23:974
Barnes DM, Bartkova J, Camplejohn RS, Gullick WJ, Smith PJ, Millis RR (1992) Overexpression of the c-erbB-2 oncoprotein: why does this occur more frequently in ductal carcinoma in situ than in invasive mammary carcinoma and is this of prognostic significance? Eur J Cancer 28:644–648
Barnes NL, Khavari S, Boland GP, Cramer A, Knox WF, Bundred NJ (2005) Absence of HER4 expression predicts recurrence of ductal carcinoma in situ of the breast. Clin Cancer Res 11:2163–2168. doi:10.1158/1078-0432.CCR-04-1633
Barros FF, Powe DG, Ellis IO, Green AR (2010) Understanding the HER family in breast cancer: interaction with ligands, dimerization and treatments. Histopathology 56:560–572. doi:10.1111/j.1365-2559.2010.03494.x
Bryan BB, Schnitt SJ, Collins LC (2006) Ductal carcinoma in situ with basal-like phenotype: a possible precursor to invasive basal-like breast cancer. Mod Pathol 19:617–621. doi:10.1038/modpathol.3800570
Burkhardt L, Grob TJ, Hermann I, Burandt E, Choschzick M, Janicke F, Muller V, Bokemeyer C, Simon R, Sauter G, Wilczak W, Lebeau A (2010) Gene amplification in ductal carcinoma in situ of the breast. Breast Cancer Res Treat 123:757–765. doi:10.1007/s10549-009-0675-8
Castellana B, Escuin D, Peiro G, Garcia-Valdecasas B, Vazquez T, Pons C, Perez-Olabarria M, Barnadas A, Lerma E (2012) ASPN and GJB2 are implicated in the mechanisms of invasion of ductal breast carcinomas. J Cancer 3:175–183. doi:10.7150/jca.4120
Doebar SC, de Monye C, Stoop H, Rothbarth J, Willemsen SP, van Deurzen CH (2016) Ductal carcinoma in situ diagnosed by breast needle biopsy: predictors of invasion in the excision specimen. Breast 27:15–21. doi:10.1016/j.breast.2016.02.014
Doebar SC, van den Broek EC, Koppert LB, Jager A, Baaijens MH, Obdeijn IM, van Deurzen CH (2016) Extent of ductal carcinoma in situ according to breast cancer subtypes: a population-based cohort study. Breast Cancer Res Treat 158:179–187. doi:10.1007/s10549-016-3862-4
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19:403–410
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC, American Society of Clinical O, College of American P (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. (unabridged version). Arch Pathol Lab Med 134:e48–e72. doi:10.1043/1543-2165-134.7.e48
Hammond ME, Hayes DF, Wolff AC (2011) Clinical notice for American Society of Clinical Oncology-College of American Pathologists guideline recommendations on ER/PgR and HER2 testing in breast cancer. J Clin Oncol 29:e458. doi:10.1200/JCO.2011.35.2245
Han K, Nofech-Mozes S, Narod S, Hanna W, Vesprini D, Saskin R, Taylor C, Kong I, Paszat L, Rakovitch E (2012) Expression of HER2neu in ductal carcinoma in situ is associated with local recurrence. Clin Oncol 24:183–189. doi:10.1016/j.clon.2011.09.008
Harvey JM, Clark GM, Osborne CK, Allred DC (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17:1474–1481
Hernandez L, Wilkerson PM, Lambros MB, Campion-Flora A, Rodrigues DN, Gauthier A, Cabral C, Pawar V, Mackay A, A’Hern R, Marchio C, Palacios J, Natrajan R, Weigelt B, Reis-Filho JS (2012) Genomic and mutational profiling of ductal carcinomas in situ and matched adjacent invasive breast cancers reveals intra-tumour genetic heterogeneity and clonal selection. J Pathol 227:42–52. doi:10.1002/path.3990
Holmes P, Lloyd J, Chervoneva I, Pequinot E, Cornfield DB, Schwartz GF, Allen KG, Palazzo JP (2011) Prognostic markers and long-term outcomes in ductal carcinoma in situ of the breast treated with excision alone. Cancer 117:3650–3657. doi:10.1002/cncr.25942
Iakovlev VV, Arneson NC, Wong V, Wang C, Leung S, Iakovleva G, Warren K, Pintilie M, Done SJ (2008) Genomic differences between pure ductal carcinoma in situ of the breast and that associated with invasive disease: a calibrated aCGH study. Clin Cancer Res 14:4446–4454. doi:10.1158/1078-0432.CCR-07-4960
Jang MH, Kim EJ, Choi Y, Lee HE, Kim YJ, Kim JH, Kang E, Kim SW, Kim IA, Park SY (2012) FGFR1 is amplified during the progression of in situ to invasive breast carcinoma. Breast Cancer Res 14:R115. doi:10.1186/bcr3239
Kerlikowske K, Molinaro AM, Gauthier ML, Berman HK, Waldman F, Bennington J, Sanchez H, Jimenez C, Stewart K, Chew K, Ljung BM, Tlsty TD (2010) Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J Natl Cancer Inst 102:627–637. doi:10.1093/jnci/djq101
Kim S, Cho N, Ryu EB, Seo M, Bae M, Chang JM, Moon WK (2014) Background Parenchymal Signal Enhancement Ratio at Preoperative MR Imaging: Association with Subsequent Local Recurrence in Patients with Ductal Carcinoma in Situ after Breast Conservation Surgery. Radiology 270:699–707
Kulkarni S, Patil DB, Diaz LK, Wiley EL, Morrow M, Khan SA (2008) COX-2 and PPARgamma expression are potential markers of recurrence risk in mammary duct carcinoma in-situ. BMC Cancer 8:36. doi:10.1186/1471-2407-8-36
Lambein K, Praet M, Forsyth R, Van den Broecke R, Braems G, Matthys B, Cocquyt V, Denys H, Pauwels P, Libbrecht L (2011) Relationship between pathological features, HER2 protein expression and HER2 and CEP17 copy number in breast cancer: biological and methodological considerations. J Clin Pathol 64:200–207. doi:10.1136/jcp.2010.084863
Lambein K, Van Bockstal M, Denys H, Libbrecht L (2014) 2013 update of the American Society of Clinical Oncology/College of American Pathologists Guideline for human epidermal growth factor receptor 2 testing: impact on immunohistochemistry-negative breast cancers. J Clin Oncol. doi:10.1200/JCO.2013.54.2530
Lambein K, Van Bockstal M, Vandemaele L, Geenen S, Rottiers I, Nuyts A, Matthys B, Praet M, Denys H, Libbrecht L (2013) Distinguishing score 0 from score 1+ in HER2 immunohistochemistry-negative breast cancer: clinical and pathobiological relevance. Am J Clin Pathol 140:561–566. doi:10.1309/AJCP4A7KTAYHZSOE
Lee S, Stewart S, Nagtegaal I, Luo J, Wu Y, Colditz G, Medina D, Allred DC (2012) Differentially expressed genes regulating the progression of ductal carcinoma in situ to invasive breast cancer. Cancer Res 72:4574–4586. doi:10.1158/0008-5472.CAN-12-0636
Lester SC, Bose S, Chen YY, Connolly JL, de Baca ME, Fitzgibbons PL, Hayes DF, Kleer C, O’Malley FP, Page DL, Smith BL, Tan LK, Weaver DL, Winer E, Members of the Cancer Committee CoAP (2009) Protocol for the examination of specimens from patients with invasive carcinoma of the breast. Arch Pathol Lab Med 133:1515–1538. doi:10.1043/1543-2165-133.10.1515
Lester SC, Bose S, Chen YY, Connolly JL, de Baca ME, Fitzgibbons PL, Hayes DF, Kleer C, O’Malley FP, Page DL, Smith BL, Weaver DL, Winer E, Members of the Cancer Committee CoAP (2009) Protocol for the examination of specimens from patients with ductal carcinoma in situ of the breast. Arch Pathol Lab Med 133:15–25. doi:10.1043/1543-2165-133.1.15
Leung CT, Brugge JS (2012) Outgrowth of single oncogene-expressing cells from suppressive epithelial environments. Nature 482:410–413. doi:10.1038/nature10826
Lin S (2007) Mixture modeling of progression pathways of heterogeneous breast tumors. J Theor Biol 249:254–261. doi:10.1016/j.jtbi.2007.08.010
Lu J, Guo H, Treekitkarnmongkol W, Li P, Zhang J, Shi B, Ling C, Zhou X, Chen T, Chiao PJ, Feng X, Seewaldt VL, Muller WJ, Sahin A, Hung MC, Yu D (2009) 14-3-3zeta cooperates with ErbB2 to promote ductal carcinoma in situ progression to invasive breast cancer by inducing epithelial-mesenchymal transition. Cancer Cell 16:195–207. doi:10.1016/j.ccr.2009.08.010
Ma XJ, Dahiya S, Richardson E, Erlander M, Sgroi DC (2009) Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res 11:R7. doi:10.1186/bcr2222
Moelans CB, van Diest PJ (2014) CEP17 copy number increase does not indicate polysomy 17. J Clin Pathol 67:454–455. doi:10.1136/jclinpath-2013-202104
Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS (2001) ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol 3:785–792. doi:10.1038/ncb0901-785
Nofech-Mozes S, Spayne J, Rakovitch E, Hanna W (2005) Prognostic and predictive molecular markers in DCIS; a review. Adv Anat Pathol 12:256–264
Nofech-Mozes S, Spayne J, Rakovitch E, Kahn HJ, Seth A, Pignol JP, Lickley L, Paszat L, Hanna W (2008) Biological markers predictive of invasive recurrence in DCIS. Clin Med Oncol 2:7–18
Noh JM, Lee J, Choi DH, Cho EY, Huh SJ, Park W, Nam SJ, Lee JE, Kil WH (2013) HER-2 overexpression is not associated with increased ipsilateral breast tumor recurrence in DCIS treated with breast-conserving surgery followed by radiotherapy. Breast 22:894–897. doi:10.1016/j.breast.2013.04.001
Park K, Han S, Kim HJ, Kim J, Shin E (2006) HER2 status in pure ductal carcinoma in situ and in the intraductal and invasive components of invasive ductal carcinoma determined by fluorescence in situ hybridization and immunohistochemistry. Histopathology 48:702–707. doi:10.1111/j.1365-2559.2006.02403.x
Perrone G, Santini D, Vincenzi B, Zagami M, La Cesa A, Bianchi A, Altomare V, Primavera A, Battista C, Vetrani A, Tonini G, Rabitti C (2005) COX-2 expression in DCIS: correlation with VEGF, HER-2/neu, prognostic molecular markers and clinicopathological features. Histopathology 46:561–568. doi:10.1111/j.1365-2559.2005.02132.x
Pradeep CR, Zeisel A, Kostler WJ, Lauriola M, Jacob-Hirsch J, Haibe-Kains B, Amariglio N, Ben-Chetrit N, Emde A, Solomonov I, Neufeld G, Piccart M, Sagi I, Sotiriou C, Rechavi G, Domany E, Desmedt C, Yarden Y (2012) Modeling invasive breast cancer: growth factors propel progression of HER2-positive premalignant lesions. Oncogene 31:3569–3583. doi:10.1038/onc.2011.547
Rakovitch E, Nofech-Mozes S, Hanna W, Narod S, Thiruchelvam D, Saskin R, Spayne J, Taylor C, Paszat L (2012) HER2/neu and Ki-67 expression predict non-invasive recurrence following breast-conserving therapy for ductal carcinoma in situ. Br J Cancer 106:1160–1165. doi:10.1038/bjc.2012.41
Rane SU, Mirza H, Grigoriadis A, Pinder SEE (2015) Selection and evolution in the genomic landscape of copy number alterations in ductal carcinoma in situ (DCIS) and its progression to invasive carcinoma of ductal/no special type: a meta-analysis Breast Cancer Res Treat 153:101–121. doi: 10.1007/s10549-015-3509-x
Ross DS, Wen YH, Brogi E (2013) Ductal carcinoma in situ: morphology-based knowledge and molecular advances. Adv Anat Pathol 20:205–2016
Ruschoff J, Lebeau A, Kreipe H, Sinn P, Gerharz CD, Koch W, Morris S, Ammann J, Untch M, Nicht-interventionelle Untersuchung HERSG (2017) Assessing HER2 testing quality in breast cancer: variables that influence HER2 positivity rate from a large, multicenter, observational study in Germany. Mod Pathol 30:217–226. doi:10.1038/modpathol.2016.164
Sauter G, Lee J, Bartlett JM, Slamon DJ, Press MF (2009) Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol 27:1323–1333. doi:10.1200/JCO.2007.14.8197
Silverstein MJ, Poller DN, Waisman JR, Colburn WJ, Barth A, Gierson ED, Lewinsky B, Gamagami P, Slamon DJ (1995) Prognostic classification of breast ductal carcinoma-in-situ. Lancet 345:1154–1157
Siziopikou KP (2013) Ductal carcinoma in situ of the breast: current concepts and future directions. Arch Pathol Lab Med 137:462–466. doi:10.5858/arpa.2012-0078-RA
Siziopikou KP, Anderson SJ, Cobleigh MA, Julian TB, Arthur DW, Zheng P, Mamounas EP, Pajon ER, Behrens RJ, Eakle JF, Leasure NC, Atkins JN, Polikoff JA, Seay TE, McCaskill-Stevens WJ, Rabinovitch R, Costantino JP, Wolmark N (2013) Preliminary results of centralized HER2 testing in ductal carcinoma in situ (DCIS): NSABP B-43. Breast Cancer Res Treat 142:415–421. doi:10.1007/s10549-013-2755-z
Siziopikou KP, Khan S (2005) Correlation of HER2 gene amplification with expression of the apoptosis-suppressing genes bcl-2 and bcl-x-L in ductal carcinoma in situ of the breast. Appl Immunohistochem Mol Morphol 13:14–18
Solin LJ, Gray R, Baehner FL, Butler SM, Hughes LL, Yoshizawa C, Cherbavaz DB, Shak S, Page DL, Sledge GW Jr, Davidson NE, Ingle JN, Perez EA, Wood WC, Sparano JA, Badve S (2013) A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst 105:701–710. doi:10.1093/jnci/djt067
Sontag L, Axelrod DE (2005) Evaluation of pathways for progression of heterogeneous breast tumors. J Theor Biol 232:179–189. doi:10.1016/j.jtbi.2004.08.002
Van Bockstal M, Lambein K, Denys H, Braems G, Nuyts A, Van den Broecke R, Cocquyt V, De Wever O, Libbrecht L (2014) Histopathological characterization of ductal carcinoma in situ (DCIS) of the breast according to HER2 amplification status and molecular subtype. Virchows Arch. doi:10.1007/s00428-014-1609-3
Van Bockstal M, Lambein K, Gevaert O, De Wever O, Praet M, Cocquyt V, Van den Broecke R, Braems G, Denys H, Libbrecht L (2013) Stromal architecture and periductal decorin are potential prognostic markers for ipsilateral locoregional recurrence in ductal carcinoma in situ of the breast. Histopathology. doi:10.1111/his.12188
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. Arch Pathol Lab Med. doi:10.5858/arpa.2013-0953-SA
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF, American Society of Clinical Oncology/College of American P (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med 131:18–43. doi:10.1043/1543-2165(2007)131[18:ASOCCO]2.0.CO;2
Acknowledgements
We thank all lab technicians of the Department of Pathology and the Center for Molecular Pathology (Ghent University Hospital) for their excellent technical assistance.
Author information
Authors and Affiliations
Contributions
KL, MVB, HD, and LL designed the study, analyzed and interpreted the data, and drafted the manuscript. RVDB, VC, and HD acquired patient data. LVDM and SG coordinated and ensured the quality of all analyses. All authors contributed to, read, and approved the final manuscript.
Corresponding author
Ethics declarations
This study has been approved by the ethics committee of Ghent University Hospital (Ghent, Belgium). The approval carries file number 2013/987 and the Belgian registration number B670201319010.
Funding
This study was funded by the ‘Klinisch onderzoeksfonds’ (clinical research fund; KL) and the ‘Speerpunt Oncologie’ (spearhead oncology; VC) from Ghent University Hospital, Ghent, Belgium.
Conflict of interest
Advisory role for Roche (KL).
The other authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Lambein, K., Van Bockstal, M., Vandemaele, L. et al. Comparison of HER2 amplification status among breast cancer subgroups offers new insights in pathways of breast cancer progression. Virchows Arch 471, 575–587 (2017). https://doi.org/10.1007/s00428-017-2161-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-017-2161-8