Abstract
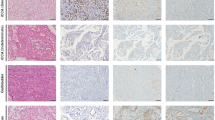
Discriminating intrahepatic cholangiocarcinoma (ICC) from hepatic metastases of pancreatic ductal adenocarcinoma (mPDAC) can be challenging. While pathologists might depend on clinical information regarding a primary tumor, their diagnosis will lead the patient either to potentially curative surgery (for ICC) or to palliation (for mPDAC). Beyond the validation of recently published potential biomarkers for PDAC (primary or metastatic) in a large cohort, we assessed diagnostic performance of the most promising candidates in the challenging task of discriminating metastatic PDAC (mPDAC) from ICC. In a training set of 87 ICC and 88 pPDAC, our previously identified biomarkers Annexin A1 (ANXA1), ANXA10, and ANXA13 were tested and compared with 11 published biomarkers or panels (MUCIN 1, Agrin, S100P, MUC5 AC, Laminin, VHL, CK 17, N-Cadherin, ELAC2, PODXL and HSPG2). Biomarkers with best results were further tested in an independent series of biopsies of 27 ICC and 36 mPDAC. Highest AUC values (between 0.72 and 0.84) for the discrimination between ICC and pPDAC were found in the training set for Annexin A1, Annexin A10, MUC5 AC, CK17, and N-Cadherin. These markers were further tested on an independent series of liver biopsies containing ICC or mPDAC. Diagnostic characteristics were evaluated for individual markers as well as for 3× panels. ANXA 10 showed the highest diagnostic potential of all single markers, correctly classifying 75% of mPDAC and 85% of ICC. Our results suggest that ANXA10 may be useful to differentiate between ICC and mPDAC, when only a tissue specimen is available.



Similar content being viewed by others
References
Bosch FX, Ribes J, Cleries R, Diaz M (2005) Epidemiology of hepatocellular carcinoma. Clinics in Liver Disease 9:191–211. doi:10.1016/j.cld.2004.12.009
Bosch FX, Ribes J, Diaz M, Cleries R (2004) Primary liver cancer: worldwide incidence and trends. Gastroenterology 127:S5–S16
Khan SA, Toledano MB, Taylor-Robinson SD (2008) Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma HPB. the official journal of the International Hepato Pancreato Biliary Association 10:77–82. doi:10.1080/13651820801992641
Bai XF, Ni XG, Zhao P, Liu SM, Wang HX, Guo B, Zhou LP, Liu F, Zhang JS, Wang K, Xie YQ, Shao YF, Zhao XH (2004) Overexpression of annexin 1 in pancreatic cancer and its clinical significance. World J Gastroenterol 10:1466–1470
Chu PG, Schwarz RE, Lau SK, Yen Y, Weiss LM (2005) Immunohistochemical staining in the diagnosis of pancreatobiliary and ampulla of Vater adenocarcinoma: application of CDX2, CK17, MUC1, and MUC2. Am J Surg Pathol 29:359–367
Hooper JE, Morgan TK, Grompe M, Sheppard BC, Troxell ML, Corless CL, Streeter PR (2012) The novel monoclonal antibody HPC2 and N-cadherin distinguish pancreatic ductal adenocarcinoma from cholangiocarcinoma. Hum Pathol 43:1583–1589. doi:10.1016/j.humpath.2011.11.012
Lok T, Chen L, Lin F, Wang HL (2014) Immunohistochemical distinction between intrahepatic cholangiocarcinoma and pancreatic ductal adenocarcinoma. Hum Pathol 45:394–400. doi:10.1016/j.humpath.2013.10.004
Lu SH, Yuan RH, Chen YL, Hsu HC, Jeng YM (2013) Annexin A10 is an immunohistochemical marker for adenocarcinoma of the upper gastrointestinal tract and pancreatobiliary system. Histopathology 63:640–648. doi:10.1111/his.12229
Ney JT, Zhou H, Sipos B, Buttner R, Chen X, Kloppel G, Gutgemann I (2007) Podocalyxin-like protein 1 expression is useful to differentiate pancreatic ductal adenocarcinomas from adenocarcinomas of the biliary and gastrointestinal tracts. Hum Pathol 38:359–364. doi:10.1016/j.humpath.2006.08.025
Padden J, Ahrens M, Kalsch J, Bertram S, Megger DA, Bracht T, Eisenacher M, Kocabayoglu P, Meyer HE, Sipos B, Baba HA, Sitek B (2015) Immunohistochemical markers distinguishing cholangiocellular carcinoma from pancreatic ductal adenocarcinoma discovered by proteomic analysis of microdissected cells. Molecular & cellular proteomics : MCP. doi: 10.1074/mcp.M115.054585
Somoracz A, Tatrai P, Horvath G, Kiss A, Kupcsulik P, Kovalszky I, Schaff Z (2010) Agrin immunohistochemistry facilitates the determination of primary versus metastatic origin of liver carcinomas. Hum Pathol 41:1310–1319. doi:10.1016/j.humpath.2009.10.029
Lazaridis KN, Gores GJ (2005) Cholangiocarcinoma. Gastroenterology 128:1655–1667
Shaib Y, El-Serag HB (2004) The epidemiology of cholangiocarcinoma. Semin Liver Dis 24:115–125. doi:10.1055/s-2004-828889
Bosman FT, World Health Organization., International Agency for Research on Cancer (2010) WHO classification of tumours of the digestive system. Chapter 10. International Agency for Research on Cancer, Lyon
Remmele W, Stegner HE (1987) Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 8:138–140
Gandou C, Harada K, Sato Y, Igarashi S, Sasaki M, Ikeda H, Nakanuma Y (2013) Hilar cholangiocarcinoma and pancreatic ductal adenocarcinoma share similar histopathologies, immunophenotypes, and development-related molecules. Hum Pathol 44:811–821. doi:10.1016/j.humpath.2012.08.004
Goldstein NS, Bassi D (2001) Cytokeratins 7, 17, and 20 reactivity in pancreatic and ampulla of vater adenocarcinomas. Percentage of positivity and distribution is affected by the cut-point threshold. Am J Clin Pathol 115:695–702. doi:10.1309/1NCM-46QX-3B5T-7XHR
McKinney KQ, Lee JG, Sindram D, Russo MW, Han DK, Bonkovsky HL, Hwang SI (2012) Identification of differentially expressed proteins from primary versus metastatic pancreatic cancer cells using subcellular proteomics. Cancer Genomics Proteomics 9:257–263
Shimizu T, Kasamatsu A, Yamamoto A, Koike K, Ishige S, Takatori H, Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H, Uzawa K (2012) Annexin A10 in human oral cancer: biomarker for tumoral growth via G1/S transition by targeting MAPK signaling pathways. PLoS One 7:e45510. doi:10.1371/journal.pone.0045510
Acknowledgement
The authors thank Laura Malkus who provided expertise that greatly assisted the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Statement
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study was supported by PROFILE consortium Ruhr, European Regional Development Fund (ERDF) objective 2 “regional Competitiveness and Employment” 2007–2013 North-Rhine-Westphalia (Germany) and by the de.NBI project (FKZ 031 A 534A) of the Bundesministerium für Bildung und Forschung (BMBF). JK received intramural research funds of the Medical Faculty of the University of Duisburg-Essen (Interne Forschungsförderung Essen, IFORES).
Rights and permissions
About this article
Cite this article
Kälsch, J., Padden, J., Bertram, S. et al. Annexin A10 optimally differentiates between intrahepatic cholangiocarcinoma and hepatic metastases of pancreatic ductal adenocarcinoma: a comparative study of immunohistochemical markers and panels. Virchows Arch 470, 537–543 (2017). https://doi.org/10.1007/s00428-017-2114-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-017-2114-2