Abstract
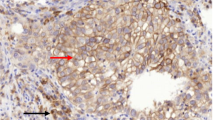
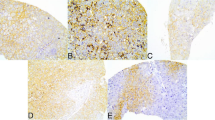
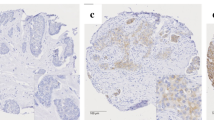
Glutamine metabolism is emerging as one aspect of dysregulated metabolism of tumors. Triple-negative breast cancer (TNBC) cells are glutamine dependent, whereas luminal-type cells tend to be glutamine independent. Therefore, TNBC patients might benefit from therapies targeting glutamine metabolism. To investigate the clinical significance of glutamine metabolism, we examined expression and prognostic significance of glutaminase in tumor cells and tumor-infiltrating lymphocytes (TILs) in TNBC. We retrieved 658 surgically resected TNBCs and analyzed glutaminase expression in tumor cells and TILs by immunohistochemical staining. Glutaminase expression was observed in 237 cases (36.0%) in tumor cells and 104 cases (15.5%) in TILs. Although glutaminase expression in tumor cells was significantly associated with a low level of TILs (p = 0.018), glutaminase expression in TILs was significantly higher in cases with a high level of TILs (p = 0.031). Glutaminase expression in tumor cells was significantly associated with poor disease-free survival in patients with lymph node metastasis and high levels of TILs (p = 0.020). In addition, it was an independent poor prognostic factor (hazard ratio = 10.643, 95% confidence interval = 1.999–56.668; p = 0.006). Glutaminase expression in tumor cells was observed in a subset of TNBC patients. It was significantly associated with a low level of TILs and poor disease-free survival in TNBCs presenting with lymph node metastasis and high levels of TILs.


Similar content being viewed by others
References
Kung HN, Marks JR, Chi JT (2011) Glutamine synthetase is a genetic determinant of cell type-specific glutamine independence in breast epithelia. PLoS Genet 7:e1002229. doi:10.1371/journal.pgen.1002229
Hsu PP, Sabatini DM (2008) Cancer cell metabolism: Warburg and beyond. Cell 134:703–707. doi:10.1016/j.cell.2008.08.021
Tennant DA, Duran RV, Gottlieb E (2010) Targeting metabolic transformation for cancer therapy. Nat Rev Cancer 10:267–277. doi:10.1038/nrc2817
Warburg O (1956) On respiratory impairment in cancer cells. Science 124:269–270
DeBerardinis RJ, Cheng T (2010) Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 29:313–324. doi:10.1038/onc.2009.358
Wise DR, Thompson CB (2010) Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci 35:427–433. doi:10.1016/j.tibs.2010.05.003
Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y (2007) Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol 178:93–105. doi:10.1083/jcb.200703099
Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB (2008) Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A 105:18782–18787. doi:10.1073/pnas.0810199105
Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV (2009) c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458:762–765. doi:10.1038/nature07823
Yang C, Sudderth J, Dang T, Bachoo RM, McDonald JG, DeBerardinis RJ (2009) Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res 69:7986–7993. doi:10.1158/0008-5472.CAN-09-2266
van den Heuvel AP, Jing J, Wooster RF, Bachman KE (2012) Analysis of glutamine dependency in non-small cell lung cancer: GLS1 splice variant GAC is essential for cancer cell growth. Cancer Biol Ther 13:1185–1194. doi:10.4161/cbt.21348
Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R, Wilson KF, Ambrosio AL, Dias SM, Dang CV, Cerione RA (2010) Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell 18:207–219. doi:10.1016/j.ccr.2010.08.009
Kaadige MR, Looper RE, Kamalanaadhan S, Ayer DE (2009) Glutamine-dependent anapleurosis dictates glucose uptake and cell growth by regulating MondoA transcriptional activity. Proc Natl Acad Sci U S A 106:14878–14883. doi:10.1073/pnas.0901221106
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, de Azambuja E, Quinaux E, Di Leo A, Michiels S, Piccart MJ, Sotiriou C (2013) Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 31:860–867. doi:10.1200/JCO.2011.41.0902
Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, Wolff AC, Wood WC, Davidson NE, Sledge GW, Sparano JA, Badve SS (2014) Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. doi:10.1200/JCO.2013.55.0491
Ahn SG, Jeong J, Hong S, Jung WH (2015) Current issues and clinical evidence in tumor-infiltrating lymphocytes in breast cancer. J Pathol Transl Med 49:355–363. doi:10.4132/jptm.2015.07.29
Mockler MB, Conroy MJ, Lysaght J (2014) Targeting T cell immunometabolism for cancer immunotherapy; understanding the impact of the tumor microenvironment. Front Oncol 4:107. doi:10.3389/fonc.2014.00107
Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, Tonc E, Schreiber RD, Pearce EJ, Pearce EL (2015) Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162:1229–1241. doi:10.1016/j.cell.2015.08.016
Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, von Torne C, Weichert W, Engels K, Solbach C, Schrader I, Dietel M, von Minckwitz G (2010) Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28:105–113. doi:10.1200/JCO.2009.23.7370
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, Perez EA, Thompson EA, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S, International TWG (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26:259–271. doi:10.1093/annonc/mdu450
Lakhani SREI, Schnitt SJ, Tan PH, van de Vijver MJ (eds) (2012) WHO classification of tumours of the breast. International Agency for Research on Cancer, Lyon
Lee HJ, Seo AN, Park SY, Kim JY, Park JY, Yu JH, Ahn JH, Gong G (2014) Low prognostic implication of fibroblast growth factor family activation in triple-negative breast cancer subsets. Ann Surg Oncol 21:1561–1568. doi:10.1245/s10434-013-3456-x
Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract 6:195–197. doi:10.1200/JOP.777003
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice guideline update. Arch Pathol Lab Med. doi:10.5858/arpa.2013-0953-SA
Kim S, Kim Do H, Jung WH, Koo JS (2013) Expression of glutamine metabolism-related proteins according to molecular subtype of breast cancer. Endocr Relat Cancer 20:339–348. doi:10.1530/ERC-12-0398
Collins CL, Wasa M, Souba WW, Abcouwer SF (1997) Regulation of glutamine synthetase in human breast carcinoma cells and experimental tumors. Surgery 122:451–463 discussion 463-454
Lee HJ, Park IA, Song IH, Shin SJ, Kim JY, Yu JH, Gong G (2016) Tertiary lymphoid structures: prognostic significance and relationship with tumour-infiltrating lymphocytes in triple-negative breast cancer. J Clin Pathol 69:422–430. doi:10.1136/jclinpath-2015-203089
Briggs KJ, Koivunen P, Cao S, Backus KM, Olenchock BA, Patel H, Zhang Q, Signoretti S, Gerfen GJ, Richardson AL, Witkiewicz AK, Cravatt BF, Clardy J, Kaelin WG Jr (2016) Paracrine induction of HIF by glutamate in breast cancer: EglN1 senses cysteine. Cell 166:126–139. doi:10.1016/j.cell.2016.05.042
Chen X, Iliopoulos D, Zhang Q, Tang Q, Greenblatt MB, Hatziapostolou M, Lim E, Tam WL, Ni M, Chen Y, Mai J, Shen H, Hu DZ, Adoro S, Hu B, Song M, Tan C, Landis MD, Ferrari M, Shin SJ, Brown M, Chang JC, Liu XS, Glimcher LH (2014) XBP1 promotes triple-negative breast cancer by controlling the HIF1alpha pathway. Nature 508:103–107. doi:10.1038/nature13119
Chaturvedi P, Gilkes DM, Wong CC, Kshitiz LW, Zhang H, Wei H, Takano N, Schito L, Levchenko A, Semenza GL (2013) Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J Clin Invest 123:189–205. doi:10.1172/JCI64993
Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM (2009) Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 457:910–914. doi:10.1038/nature07762
Vander Heiden MG (2011) Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov 10:671–684. doi:10.1038/nrd3504
Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812. doi:10.1126/science.1164382
Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, Marks KM, Prins RM, Ward PS, Yen KE, Liau LM, Rabinowitz JD, Cantley LC, Thompson CB, Vander Heiden MG, Su SM (2009) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462:739–744. doi:10.1038/nature08617
Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, Cross JR, Fantin VR, Hedvat CV, Perl AE, Rabinowitz JD, Carroll M, Su SM, Sharp KA, Levine RL, Thompson CB (2010) The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 17:225–234. doi:10.1016/j.ccr.2010.01.020
Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, Li Y, Bhagwat N, Vasanthakumar A, Fernandez HF, Tallman MS, Sun Z, Wolniak K, Peeters JK, Liu W, Choe SE, Fantin VR, Paietta E, Lowenberg B, Licht JD, Godley LA, Delwel R, Valk PJ, Thompson CB, Levine RL, Melnick A (2010) Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 18:553–567. doi:10.1016/j.ccr.2010.11.015
Swamy M, Pathak S, Grzes KM, Damerow S, Sinclair LV, van Aalten DM, Cantrell DA (2016) Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nat Immunol 17:712–720. doi:10.1038/ni.3439
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2015R1C1A1A02036484) and the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea (2016-732).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Institutional Review Board of Asan Medical Center and confirmed to the provisions of the Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Joo Young Kim and Sun-Hee Heo contributed equally to this article.
Rights and permissions
About this article
Cite this article
Kim, J.Y., Heo, SH., Choi, S.K. et al. Glutaminase expression is a poor prognostic factor in node-positive triple-negative breast cancer patients with a high level of tumor-infiltrating lymphocytes. Virchows Arch 470, 381–389 (2017). https://doi.org/10.1007/s00428-017-2083-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-017-2083-5