Abstract
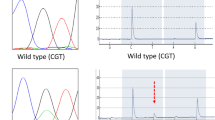
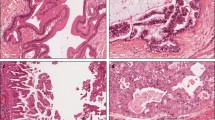
In contrast to pancreatic ductal adenocarcinomas (PDAs), intraductal papillary mucinous neoplasms (IPMNs) frequently harbour GNAS mutations. To characterise GNAS-mutated pancreatic carcinomas, we examined mutations of GNAS and KRAS in 290 pancreatic adenocarcinomas and 77 pancreatic intraepithelial neoplasias (PanINs). In 64 % (39/61) of IPMNs and 37 % (11/30) of IPMN-associated adenocarcinomas, a GNAS mutation was found. GNAS mutations were frequent (78 %, 7/9) in mucinous carcinomas, with or without associated IPMN. In contrast, GNAS mutations were rarely observed in PDAs (1 %, 1/88) and PanINs (3 %, 2/77), and not at all in mucinous cystic neoplasms (MCNs) (0/10), neuroendocrine neoplasms (0/52), acinar cell neoplasms (0/16), serous cystadenomas (0/10), and solid-pseudopapillary neoplasms (0/14). We found GNAS mutations in 55/91 IPMNs with or without associated invasive carcinoma, solely in intestinal-type (78 %, 21/27) and gastric-type (62 %, 34/55) IPMNs. Of the IPMN-associated adenocarcinomas, mucinous-subtype tumours harboured GNAS mutations more frequently (83 %, 5/6) than tubular-subtype tumours (25 %, 6/24) (p = 0.02). We separately analysed GNAS in the adenocarcinoma and the IPMN component in the IPMN-associated adenocarcinomas. In all mucinous-subtype tumours, the two components exhibited identical genotypes. In contrast, the two components in 8 of 24 tubular-subtype tumours exhibited different genotypes, indicating intratumour heterogeneity. In conclusion, mucinous carcinomas with or without associated IPMN as well as IPMNs frequently harbour a GNAS mutation, reinforcing the notion that these constitute a spectrum of pancreatic tumours. Clinically and pathologically, these tumours are associated, but GNAS mutation sheds further light on this spectrum.

Similar content being viewed by others
References
Weinstein LS, Liu J, Sakamoto A, Xie T, Chen M (2004) Minireview: GNAS: normal and abnormal functions. Endocrinology 145(12):5459–5464. doi:10.1210/en.2004-0865
O’Hayre M, Vazquez-Prado J, Kufareva I, Stawiski EW, Handel TM, Seshagiri S, Gutkind JS (2013) The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer 13(6):412–424. doi:10.1038/nrc3521
Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L (1989) GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature 340(6236):692–696. doi:10.1038/340692a0
Lyons J, Landis CA, Harsh G, Vallar L, Grunewald K, Feichtinger H, Duh QY, Clark OH, Kawasaki E, Bourne HR et al (1990) Two G protein oncogenes in human endocrine tumors. Science 249(4969):655–659
Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM (1991) Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med 325(24):1688–1695. doi:10.1056/nejm199112123252403
Okamoto S, Hisaoka M, Ushijima M, Nakahara S, Toyoshima S, Hashimoto H (2000) Activating Gs(alpha) mutation in intramuscular myxomas with and without fibrous dysplasia of bone. Virchows Arch 437(2):133–137
Fragoso MC, Latronico AC, Carvalho FM, Zerbini MC, Marcondes JA, Araujo LM, Lando VS, Frazzatto ET, Mendonca BB, Villares SM (1998) Activating mutation of the stimulatory G protein (gsp) as a putative cause of ovarian and testicular human stromal Leydig cell tumors. J Clin Endocrinol Metab 83(6):2074–2078
Delaney D, Diss TC, Presneau N, Hing S, Berisha F, Idowu BD, O’Donnell P, Skinner JA, Tirabosco R, Flanagan AM (2009) GNAS1 mutations occur more commonly than previously thought in intramuscular myxoma. Mod Pathol 22(5):718–724. doi:10.1038/modpathol.2009.32
Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH, Wolfgang CL, Klein AP, Diaz LA Jr, Allen PJ, Schmidt CM, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B (2011) Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 3(92):92ra66. doi:10.1126/scitranslmed.3002543
Furukawa T, Kuboki Y, Tanji E, Yoshida S, Hatori T, Yamamoto M, Shibata N, Shimizu K, Kamatani N, Shiratori K (2011) Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep 1:161. doi:10.1038/srep00161
Matsubara A, Sekine S, Kushima R, Ogawa R, Taniguchi H, Tsuda H, Kanai Y (2013) Frequent GNAS and KRAS mutations in pyloric gland adenoma of the stomach and duodenum. J Pathol 229(4):579–587. doi:10.1002/path.4153
Yamada M, Sekine S, Ogawa R, Taniguchi H, Kushima R, Tsuda H, Kanai Y (2012) Frequent activating GNAS mutations in villous adenoma of the colorectum. J Pathol 228(1):113–118. doi:10.1002/path.4012
Nishikawa G, Sekine S, Ogawa R, Matsubara A, Mori T, Taniguchi H, Kushima R, Hiraoka N, Tsuta K, Tsuda H, Kanai Y (2013) Frequent GNAS mutations in low-grade appendiceal mucinous neoplasms. Br J Cancer 108(4):951–958. doi:10.1038/bjc.2013.47
Tsai JH, Yuan RH, Chen YL, Liau JY, Jeng YM (2013) GNAS is frequently mutated in a specific subgroup of intraductal papillary neoplasms of the bile duct. Am J Surg Pathol 37(12):1862–1870. doi:10.1097/PAS.0b013e3182986bb5
Sasaki M, Matsubara T, Nitta T, Sato Y, Nakanuma Y (2013) GNAS and KRAS mutations are common in intraductal papillary neoplasms of the bile duct. PLoS ONE 8(12):e81706. doi:10.1371/journal.pone.0081706
Matsubara A, Sekine S, Ogawa R, Yoshida M, Kasamatsu T, Tsuda H, Kanai Y (2013) Lobular endocervical glandular hyperplasia is a neoplastic entity with frequent activating GNAS mutations. Am J Surg Pathol. doi:10.1097/pas.0000000000000093
Bourne HR, Landis CA, Masters SB (1989) Hydrolysis of GTP by the alpha-chain of Gs and other GTP binding proteins. Proteins 6(3):222–230. doi:10.1002/prot.340060304
Wilson CH, McIntyre RE, Arends MJ, Adams DJ (2010) The activating mutation R201C in GNAS promotes intestinal tumourigenesis in Apc(Min/+) mice through activation of Wnt and ERK1/2 MAPK pathways. Oncogene 29(32):4567–4575. doi:10.1038/onc.2010.202
Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, Hruban RH, Maitra A, Kinzler K, Vogelstein B, Goggins M (2012) Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 142(4):730–733.e739. doi:10.1053/j.gastro.2011.12.042
Grutzmann R, Niedergethmann M, Pilarsky C, Kloppel G, Saeger HD (2010) Intraductal papillary mucinous tumors of the pancreas: biology, diagnosis, and treatment. Oncologist 15(12):1294–1309. doi:10.1634/theoncologist. 2010-0151
Hong SM, Park JY, Hruban RH, Goggins M (2011) Molecular signatures of pancreatic cancer. Arch Pathol Lab Med 135(6):716–727. doi:10.1043/2010-0566-ra.1
Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) WHO classification of tumours of the digestive system. International Agency for Research on Cancer, Lyon
Furukawa T, Kloppel G, Volkan Adsay N, Albores-Saavedra J, Fukushima N, Horii A, Hruban RH, Kato Y, Klimstra DS, Longnecker DS, Luttges J, Offerhaus GJ, Shimizu M, Sunamura M, Suriawinata A, Takaori K, Yonezawa S (2005) Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch 447(5):794–799. doi:10.1007/s00428-005-0039-7
Basturk O, Khayyata S, Klimstra DS, Hruban RH, Zamboni G, Coban I, Adsay NV (2010) Preferential expression of MUC6 in oncocytic and pancreatobiliary types of intraductal papillary neoplasms highlights a pyloropancreatic pathway, distinct from the intestinal pathway, in pancreatic carcinogenesis. Am J Surg Pathol 34(3):364–370. doi:10.1097/PAS.0b013e3181cf8bb6
Yatabe Y, Hida T, Horio Y, Kosaka T, Takahashi T, Mitsudomi T (2006) A rapid, sensitive assay to detect EGFR mutation in small biopsy specimens from lung cancer. J Mol Diagn 8(3):335–341. doi:10.2353/jmoldx.2006.050104
Hosoda W, Takagi T, Mizuno N, Shimizu Y, Sano T, Yamao K, Yatabe Y (2010) Diagnostic approach to pancreatic tumors with the specimens of endoscopic ultrasound-guided fine needle aspiration. Pathol Int 60(5):358–364. doi:10.1111/j.1440-1827.2010.02527.x
Pao W, Ladanyi M (2007) Epidermal growth factor receptor mutation testing in lung cancer: searching for the ideal method. Clin Cancer Res 13(17):4954–4955. doi:10.1158/1078-0432.ccr-07-1387
Mino-Kenudson M, Fernandez-del Castillo C, Baba Y, Valsangkar NP, Liss AS, Hsu M, Correa-Gallego C, Ingkakul T, Perez Johnston R, Turner BG, Androutsopoulos V, Deshpande V, McGrath D, Sahani DV, Brugge WR, Ogino S, Pitman MB, Warshaw AL, Thayer SP (2011) Prognosis of invasive intraductal papillary mucinous neoplasm depends on histological and precursor epithelial subtypes. Gut 60(12):1712–1720. doi:10.1136/gut.2010.232272
Poultsides GA, Reddy S, Cameron JL, Hruban RH, Pawlik TM, Ahuja N, Jain A, Edil BH, Iacobuzio-Donahue CA, Schulick RD, Wolfgang CL (2010) Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg 251(3):470–476. doi:10.1097/SLA.0b013e3181cf8a19
Yamaguchi K, Kanemitsu S, Hatori T, Maguchi H, Shimizu Y, Tada M, Nakagohri T, Hanada K, Osanai M, Noda Y, Nakaizumi A, Furukawa T, Ban S, Nobukawa B, Kato Y, Tanaka M (2011) Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas 40(4):571–580. doi:10.1097/MPA.0b013e318215010c
Ideno N, Ohtsuka T, Kono H, Fujiwara K, Oda Y, Aishima S, Ito T, Ishigami K, Tokunaga S, Ohuchida K, Takahata S, Nakamura M, Mizumoto K, Tanaka M (2013) Intraductal papillary mucinous neoplasms of the pancreas with distinct pancreatic ductal adenocarcinomas are frequently of gastric subtype. Ann Surg 258(1):141–151. doi:10.1097/SLA.0b013e31828cd008
Koh YX, Chok AY, Zheng HL, Tan CS, Goh BK (2014) Systematic review and meta-analysis comparing the surgical outcomes of invasive intraductal papillary mucinous neoplasms and conventional pancreatic ductal adenocarcinoma. Ann Surg Oncol 21(8):2782–2800. doi:10.1245/s10434-014-3639-0
Tanaka M, Fernandez-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K (2012) International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 12(3):183–197. doi:10.1016/j.pan.2012.04.004
Amato E, Molin MD, Mafficini A, Yu J, Malleo G, Rusev B, Fassan M, Antonello D, Sadakari Y, Castelli P, Zamboni G, Maitra A, Salvia R, Hruban RH, Bassi C, Capelli P, Lawlor RT, Goggins M, Scarpa A (2014) Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol 233(3):217–227. doi:10.1002/path.4344
Matsuno S, Egawa S, Fukuyama S, Motoi F, Sunamura M, Isaji S, Imaizumi T, Okada S, Kato H, Suda K, Nakao A, Hiraoka T, Hosotani R, Takeda K (2004) Pancreatic cancer registry in Japan: 20 years of experience. Pancreas 28(3):219–230
Iacobuzio-Donahue CA, Klimstra DS, Adsay NV, Wilentz RE, Argani P, Sohn TA, Yeo CJ, Cameron JL, Kern SE, Hruban RH (2000) Dpc-4 protein is expressed in virtually all human intraductal papillary mucinous neoplasms of the pancreas: comparison with conventional ductal adenocarcinomas. Am J Pathol 157(3):755–761. doi:10.1016/s0002-9440(10)64589-0
Ueki T, Toyota M, Sohn T, Yeo CJ, Issa JP, Hruban RH, Goggins M (2000) Hypermethylation of multiple genes in pancreatic adenocarcinoma. Cancer Res 60(7):1835–1839
House MG, Guo M, Iacobuzio-Donahue C, Herman JG (2003) Molecular progression of promoter methylation in intraductal papillary mucinous neoplasms (IPMN) of the pancreas. Carcinogenesis 24(2):193–198
Adsay NV, Merati K, Basturk O, Iacobuzio-Donahue C, Levi E, Cheng JD, Sarkar FH, Hruban RH, Klimstra DS (2004) Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol 28(7):839–848
Dal Molin M, Matthaei H, Wu J, Blackford A, Debeljak M, Rezaee N, Wolfgang CL, Butturini G, Salvia R, Bassi C, Goggins MG, Kinzler KW, Vogelstein B, Eshleman JR, Hruban RH, Maitra A (2013) Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg Oncol 20(12):3802–3808. doi:10.1245/s10434-013-3096-1
Seidel G, Zahurak M, Iacobuzio-Donahue C, Sohn TA, Adsay NV, Yeo CJ, Lillemoe KD, Cameron JL, Hruban RH, Wilentz RE (2002) Almost all infiltrating colloid carcinomas of the pancreas and periampullary region arise from in situ papillary neoplasms: a study of 39 cases. Am J Surg Pathol 26(1):56–63
Acknowledgments
The authors thank Chieko Yamada, Noriko Shibata, Ayako Nonaka, and Motoko Nimura for their excellent technical assistance with the molecular genetics. This work was supported in part by a Grant-in-Aid (B-25293090) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosoda, W., Sasaki, E., Murakami, Y. et al. GNAS mutation is a frequent event in pancreatic intraductal papillary mucinous neoplasms and associated adenocarcinomas. Virchows Arch 466, 665–674 (2015). https://doi.org/10.1007/s00428-015-1751-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-015-1751-6