Abstract
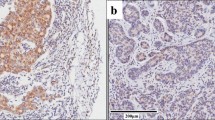
Poor prognosis in ovarian high-grade serous carcinoma (HGSC) is largely related to resistance to chemotherapy. Tumour hypoxia is known to be associated with chemotherapy resistance. Stabilisation of hypoxia-inducible factor-1α upregulates the expression of downstream genes such as carbonic anhydrase 9 (CA9) and vascular endothelial growth factor (VEGF). This study was undertaken to analyse the hypoxia profile as indicated by the co-expression of VEGF and CA9 and its correlation with survival. VEGF and CA9 expressions were examined in tissue microarray of 97 cases of ovarian high-grade serous carcinoma using immunohistochemistry. High expression of either VEGF or CA9, individually, was associated with decreased overall survival (p = 0.006 and p = 0.05 respectively). Combined high expression of both markers, to give a ‘hypoxia profile’, was associated with chemotherapy resistance (p = 0.036) and showed worse overall survival with a significant p value (p = 0.001). Using multivariate analysis, hypoxia profile was an independent prognostic factor for overall survival (p = 0.028). The combined high expression CA9 and VEGF phenotype, described as high hypoxia profile group, was significantly associated with increased resistance to chemotherapy and poor overall survival. This group may benefit from combined targeted therapy for effective response in ovarian HGSC.


Similar content being viewed by others
References
Westfall D, Roma AA, Silva EG (2009) High-grade serous carcinoma of the ovary. Ann Diagn Pathol 13(4):285–290
Burger RA (2011) Overview of anti-angiogenic agents in development for ovarian cancer. Gynecol Oncol 121:230–238
Semenza GL (2001) Regulation of hypoxia-induced angiogenesis: a chaperone escorts VEGF to the dance. J Clin Invest 108:39–40
Ziello JE, Jovin IS, Huang Y (2007) Hypoxia-inducible factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med 80(2):51–60
Ruan K, Song G, Ouyang G (2009) Role of hypoxia in the hallmarks of human cancer. J Cell Biochem 107:1053–1062
Wykoff CC, Beasley NJ, Watson PH et al (2000) Hypoxia-inducible expression of tumour-associated carbonic anhydrases. Cancer Res 60:7075–7083
Shannon AM, Bouchier-Hayes DJ, Condron CM et al (2003) Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev 29(4):297–307
Potter CPS, Harris AL (2003) Diagnostic, prognostic and therapeutic implications of carbonic anhydrases in cancer. Br J Cancer 89:2–7
Hockel M, Schlenger K, Aral B et al (1996) Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res 56:4509–4515
Brizel DM, Scully SP, Harrelson JM et al (1996) Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res 56:941–943
Mahoney BP, Raghunand N, Baggett B et al (2003) Tumor acidity, ion trapping and chemotherapeutics I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem Pharmacol 66:1207–1218
Hussain SA, Ganesan R, Reynolds G et al (2007) Hypoxia-regulated carbonic anhydrase IX expression is associated with poor survival in patients with invasive breast cancer. Br J Cancer 96(1):104–109
Loncaster JA, Harris AL, Davidson SE et al (2001) Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumour oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res 61(17):6394–6399
Kim SJ, Rabbani ZN, Dewhirst MW et al (2005) Expression of HIF-1α, CA IX, VEGF and MMP-9 in surgically resected non-small cell lung cancer. Lung Cancer 49(3):325–335
Driessen A, Landuyt W, Pastorekova S et al (2006) Expression of carbonic anhydrase IX (CA IX), a hypoxia-related protein, rather than vascular-endothelial growth factor (VEGF), a pro-angiogenic factor, correlates with an extremely poor prognosis in esophageal and gastric adenocarcinomas. Ann Surg 243:334–340
Hui EP, Chan ATC, Pezzella F et al (2002) Coexpression of hypoxia-inducible factors 1α and 2α, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res 8:2595–2604
Hynninen P, Vaskivuo L, Saarnio J et al (2006) Expression of transmembrane carbonic anhydrases IX and XII in ovarian tumours. Histopathology 49:594–602
Eisenhauer EA, Therasse P, Bagaerts J et al (2009) New response evaluation criteria in solid tumors: revised RECIT guideline (Version 1.1). Eur J Cancer 45:228–247
Markman M, Hoskins W (1992) Responses to salvage chemotherapy in ovarian cancer: a critical need for precise definitions of the treated population. J Clin Oncol 10(4):513–514
Rosen DG, Huang X, Deavers MT et al (2004) Validation of tissue microarray technology in ovarian carcinoma. Mod Pathol 17:790–797
Camp RL, Charette LA, Rimm DL (2000) Validation of tissue microarray technology in breast carcinoma. Lab Invest 80(12):1943–1949
Kononen J, Bubendorf L, Kallioniemi A et al (1998) Tissue microarrays for high-throughput molecular profiling of tumour specimens. Nat Med 4:844–847
Shibata D, Martin WJ, Arnheim N (1988) Analysis of DNA sequences in forty-year-old paraffin-embedded thin-tissue sections: a bridge between molecular biology and classical histology. Cancer Res 48:4564–4566
McCarty KS Jr, Miller LS, Cox EB et al (1985) Estrogen receptor analyses. Correlation of biochemical and immuno-histochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med 109:716–721
Camp RL, Dolled-Filhart M, Rimm DL (2004) X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10:7252–7259
Phuoc NB, Ehara H, Gotoh T et al (2008) Prognostic value of the co-expression of carbonic anhydrase IX and vascular endothelial growth factor in patients with clear cell renal cell carcinoma. Oncol Rep 20:525–530
Duncan TJ, Al-Attar A, Rolland P et al (2008) Vascular endothelial growth factor expression in ovarian cancer: a model for targeted use of novel therapies? Clin Cancer Res 14(10):3030–3035
Greenberg S, Rugo HS (2010) Triple-negative breast cancer: role of antiangiogenic agents. Cancer J 16(1):33–38
Sivridis E, Giatromanolaki A, Gatter KC et al (2002) Association of hypoxia-inducible factors 1α and 2α with activated angiogenic pathways and prognosis in patients with endometrial carcinoma. Cancer 95(5):1055–63
Saarnio J, Parkkila S, Parkkila A et al (1998) Immunohistochemical study of colorectal tumour for expression of a novel transmembrane carbonic anhydrase MN/CA IX, with potential value as a marker of cell proliferation. Am J Pathol 153(1):279–285
Birner P, Schindl M, Obermair A et al (2001) Expression of hypoxia-inducible factor 1a in epithelial ovarian tumors: its impact on prognosis and on response to chemotherapy. Clin Cancer Res 7:1661–1668
Wouters BG, Weppler SA, Koritzinsky M et al (2002) Hypoxia as a target for combined modality treatments. Eur J Cancer 38:240–257
Jubb AM, Pham TQ, Hanby AM et al (2004) Expression of vascular endothelial growth factor, hypoxia inducible factor 1alpha, and carbonic anhydrase IX in human tumours. J Clin Pathol 57:504–512
Conflict of interest
The authors declare they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Williams, E., Martin, S., Moss, R. et al. Co-expression of VEGF and CA9 in ovarian high-grade serous carcinoma and relationship to survival. Virchows Arch 461, 33–39 (2012). https://doi.org/10.1007/s00428-012-1252-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-012-1252-9