Abstract
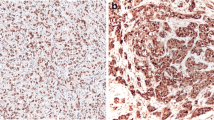
Tubulin is one of the molecular components that regulate cytosketal structure relating to cell differentiation, invasion, and metastasis in cancer. Recently, glu-tubulin, in which the C-terminal tyrosine of α-tubulin is removed by tubulin carboxypeptidase, overexpression has been reported in malignant tumors of the mammary gland immunohistochemically. We identified 147 cases accessioned in the Department of Pathology, International University of Health and Welfare Hospital from 2003 to 2009. Of the 78 malignant tumor cases, staining for glu-tubulin was observed in 56 (71.8%), and only 22 cases showed no significant staining. However, in benign disease, glu-tubulin staining was detected in the cytoplasm of fibroblasts and endothelial cells, but was completely absent from epithelial cells in 64 of 69 cases. When the expression of glu-tubulin was compared between malignant tumor, benign tumor, and other benign disease, a significant differentiation was found among expressions of this protein. These results indicate that glu-tubulin represents a strong selective expression for cancer cells and may be useful to identify and quantify human breast cancer.


Similar content being viewed by others
References
Rodriguez OC, Schaefer AW, Mandato CA et al (2003) Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol 5:599–609
Kavallaris M, Kuo DY, Burkhart CA et al (1997) Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest 100:1282–1293
Carles G, Braguer D, Dumontet C et al (1999) Differentiation of human colon cancer cells changes the expression of beta-tubulin isotypes and MAPs. Br J Cancer 80:1162–1168
Sève P, Isaac S, Trédan O et al (2005) Expression of class III β-tubulin is predictive of patient outcome in patients with non-small cell lung cancer receiving vinorelbine-based chemotherapy. Clin Cancer Res 11:5481–5486
Terry S, Ploussard G, Allory Y et al (2009) Increased expression of class III beta-tubulin in castration-resistant human prostate cancer. Br J Cancer 101:951–956
Bernard-Marty C, Treilleux I, Dumontet C et al (2002) Microtubule-associated parameters as predictive markers of docetaxel activity in advanced breast cancer patients: results of a pilot study. Clin Breast Cancer 3:341–345
Mialhe A, Lafanechère L, Treilleux I et al (2001) Tubulin detyrosination is a frequent occurrence in breast cancers of poor prognosis. Cancer Res 61:5024–5027
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19:403–410
Gundersen GG, Bulinski JC (1986) Microtubule arrays in differentiated cells contain elevated levels of a post-translationally modified form of tubulin. Eur J Cell Biol 42:288–294
Kreis TE (1987) Microtubules containing detyrosinated tubulin are less dynamic. EMBO J 6:2597–2606
Schulze E, Asai DJ, Bulinski JC et al (1987) Posttranslational modification and microtubule stability. J Cell Biol 105:2167–2177
Prescott AR, Vestberg M, Warn RM (1989) Microtubules rich in modified alpha-tubulin characterize the tail processes of motile fibroblasts. J Cell Sci 94:227–236
Wolff AC, Hammond ME, Schwartz JN et al (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145
Wittmann T, Waterman-Storer CM (2001) Cell motility: can Rho GTPases and microtubules point the way? J Cell Sci 114:3795–3803
Nabi IR (1999) The polarization of the motile cell. J Cell Sci 112:1803–1811
Zheng Y (2004) G protein control of microtubule assembly. Annu Rev Cell Dev Biol 20:867–894
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4:253–265
Gundersen GG, Bulinski JC (1988) Selective stabilization of microtubules oriented toward the direction of cell migration. Proc Natl Acad Sci USA 85:5946–5950
Wen Y, Eng CH, Schmoranzer J et al (2004) EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol 6:820–830
Acknowledgements
The authors thank Dr. Kanji Sugiyama (Department of Anatomy, Meikai University School of Dentistry) for technical assistance with immunohistochemical staining and Dr. Megumi Takehara (Department of Surgery, Jichi Medical University) for her help and support in obtaining the clinical data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuroda, H., Saito, K., Kuroda, M. et al. Differential expression of glu-tubulin in relation to mammary gland disease. Virchows Arch 457, 477–482 (2010). https://doi.org/10.1007/s00428-010-0955-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-010-0955-z