Abstract
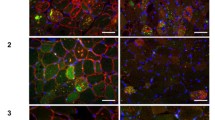
Limb girdle muscular dystrophy type 2I (LGMD2I) is due to mutations in the fukutin-related protein gene (FKRP), encoding a putative glycosyltransferase involved in α-dystroglycan processing. To further characterize the molecular pathogenesis of LGMD2I, we conducted a histological, immunohistochemical, ultrastructural and molecular analysis of ten muscle biopsies from patients with molecularly diagnosed LGMD2I. Hypoglycosylation of α-dystroglycan was observed in all FKRP-mutated patients. Muscle histopathology was consistent with either severe muscular dystrophy or myopathy with a mild inflammatory response consisting of up-regulation of class I major histocompatibility complex in skeletal muscle fibers and small foci of mononuclear cells. At the ultrastructural level, muscle fibers showed focal thinning of basal lamina and swollen endoplasmic reticulum cisternae with membrane re-arrangement. The pathways of the unfolded protein response (UPR; glucose-regulated protein 78 and CHOP) were significantly activated in LGMD2I muscle tissue. Our data suggest that the UPR response is activated in LGMD2I muscle biopsies, and the observed histopathological and ultrastructural alterations may be related to sarcoplasmic structures involved in FKRP and α-dystroglycan metabolism and malfunctioning.




Similar content being viewed by others
References
Averous J, Bruhat A, Jousse C, Carraro V, Thiel G, Fafournoux P (2004) Induction of CHOP expression by amino acid limitation requires both ATF4 expression and ATF2 phosphorylation. J Biol Chem 279:5288–5297
Beedle AM, Nienaber PM, Campbell KP (2007) Fukutin-related protein associates with the sarcolemmal dystrophin-glycoprotein complex. J Biol Chem 282:16713–16717
Bernales S, McDonald KL, Walter P (2006) Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLos Biol 4:E423
Boito CA, Melacini P, Vianello A, Prandini P, Gavassini BF, Bagattin A, Siciliano G, Angelini C, Pegoraro (2005) Clinical and molecular characterization in limb girdle muscular dystrophy 2I patients. Arch Neurol 62:1894–1899
Brockington M, Blake DJ, Prandini P, Brown SC, Torelli S, Benson MA, Ponting CP, Estournet B, Romero NB, Mercuri E, Voit T, Sewry CA, Guicheney P, Muntoni F (2001) Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin α2 deficiency and abnormal glycosylation of α-dystroglycan. Am J Hum Genet 69:1198–1209
Brockington M, Yuva Y, Prandini P, Brown SC, Torelli S, Benson MA, Herrmann R, Anderson LV, Bashir R, Burgunder JM, Fallet S, Romero N, Fardeau M, Straub V, Storey G, Pollitt C, Richard I, Sewry CA, Bushby K, Voit T, Blake DJ, Muntoni F (2001) Mutations in the fukutin-related protein gene (FKRP) identify limb girdle muscular dystrophy 2I as a milder allelic variant of congenital muscular dystrophy MDC1C. Hum Mol Genet 10:2851–2859
Cenacchi G, Fanin M, De Giorgi LB, Angelini C (2005) Ultrastructural changes in dysferlinopathy support detective membrane repair mechanism. J Clin Pathol 58:190–195
Cox S, Chapman RE, Walter P (1997) The unfolded protein response coordinates the production of endoplasmic reticulum protein and endoplasmic reticulum membrane. Mol Biol Cell 8:1805–1814
de Paula F, Vieira N, Starling A, Yamamoto LU, Lima B, de Cassia Pavanello R, Vainzof M, Nigro V, Zatz M (2003) Asymptomatic carrier for homozygous novel mutations in the FKRP gene: the other end of the spectrum. Eur J Hum Genet 11:923–930
Dolatshad NF, Brockington M, Torelli S, Skordis L, Wewer U, Wells DJ, Muntoni F, Brown SC (2005) Mutated fukutin-related protein (FKRP) localises as wild type in differentiated muscle cells. Exp Cell Res 309:370–378
Ellgaard L, Helenius A (2001) ER quality control: towards an understanding at the molecular level. Curr Opin Cell Biol 13:431–437
Ervasti JM, Campbell KP (1991) Membrane organization of the dystrophin-glycoprotein complex. Cell 66:1121–1131
Esapa CT, Benson MA, Schroder JE, Martin-Rendon E, Brockington M, Brown SC, Muntoni F, Kroger S, Blake DJ (2002) Functional requirements for fukutin-related protein in the Golgi apparatus. Hum Mol Genet 11:3319–3331
Esapa CT, McIlhinney RA, Blake DJ (2005) Fukutin-related protein mutations that cause congenital muscular dystrophy result in ER-retention of the mutant protein in cultured cells. Hum Mol Genet 14:295–305
Gardner-Medwin D, Walton JN (1969) In Disorders of Voluntary Muscle. In: Walton JN Churchill, London
Henry MD, Campbell KP (1998) A role for dystroglycan in basement membrane assembly. Cell 95:859–870
Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP (1992) Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 355:696–702
Keramaris-Vrantsis E, Lu PJ, Doran T, Zillmer A, Ashar J, Esapa CT, Benson MA, Blake DJ, Rosenfeld J, Lu QL (2007) Fukutin-related protein localizes to the Golgi apparatus and mutations lead to mislocalization in muscle in vivo. Muscle Nerve 36:455–465
Martin PT (2006) Mechanisms of disease: congenital muscular dystrophies-glycosylation takes center stage. Nat Clin Pract Neurol 2:222–230
Matsumoto M, Minami M, Takeda K, Sakao Y, Akira S (1996) Ectopic expression of CHOP (GADD153) induces apoptosis in M1 myeloblastic leukemia cells. FEBS Lett 395:143–147
Matsumoto H, Noguchi S, Sugie K, Ogawa M, Murayama K, Hayashi YK, Nishino I (2004) Subcellular localization of fukutin and fukutin-related protein in muscle cells. J Biochem (Tokyo) 135:709–712
Mercuri E, Brockington M, Straub V, Ouijano-Roy S, Yuva Y, Herrmann R, Brown SC, Torelli S, Dubowitz V, Blake DJ, Romero NB, Estournet B, Sewry CA, Guicheney P, Voit T, Muntoni F (2002) Phenotypic spectrum associated with mutations in the fukutin-related protein gene. Ann Neurol 53:537–542
Mori K (2000) Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell 101:451–454
Nagaraju K, Casciola-Raben L, Lundberg I, Rawat R, Cutting S, Thapliyal R, Chang J, Divedi S, Mitsak M, Chen YW, Plotz P, Rosen A, Hoffman EP, Raben N (2005) Activation of the endoplasmic reticulum stress response in autoimmune myositis. Arthritis Rheum 52:1824–1835
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98–103
Sriburi R, Jackowski S, Mori K, Brewer JW (2004) XBP1: A link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol 167:35–41
Torelli S, Brown SC, Brockington M, Dolatshad NF, Jimenez C, Skordis L, Feng LH, Merlini L, Jones DH, Romero N, Wewer U, Voit T, Sewry CA, Noguchi S, Nishino I, Muntoni F (2005) Sub-cellular localization of fukutin related protein in different cell lines and in the muscle of patients with MDC1C and LGMD2I. Neuromuscul Disord 15:836–843
Walter MC, Petersen JA, Stucka R, Fischer D, Schroder R, Vorgerd M, Schroers A, Schreiber H, Hanemann CO, Knirsch U, Rosenbohm A, Huebner A, Barisic N, Horvath R, Komoly S, Reilich P, Muller-Felber W, Pongratz D, Muller JS, Auerswald EA, Lochmuller H (2004) FKRP (826C>A) frequently causes limb-girdle muscular dystrophy in German patients. J Med Genet 41:E50
Xing X, Lai M, Wang Y, Xu E, Huang O (2006) Overexpression of glucose-regulated protein 78 in colon cancer. Clin Chim Acta 364:308–315
Acknowledgment
This study was supported by a grant from the Italian Telethon (number GTF05003) and EuroBioBank.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boito, C.A., Fanin, M., Gavassini, B.F. et al. Biochemical and ultrastructural evidence of endoplasmic reticulum stress in LGMD2I. Virchows Arch 451, 1047–1055 (2007). https://doi.org/10.1007/s00428-007-0515-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-007-0515-3