Abstract
Primary retroperitoneal cystadenomas are extremely rare. This is the first report in literature to describe a primary retroperitoneal cystadenoma with a sarcoma-like mural nodule. A 45-year-old woman complained of a left-sided abdominal mass. A computed tomography scan revealed a cystic mass with a mural nodule, which seemed to originate from the tail of the pancreas. At laparotomy the cyst was not adhered to the pancreas but localized retroperitoneally. Histologic examination showed a mucinous cystadenoma with only foci of borderline malignancy with a mural “sarcoma-like” nodule. In view of the surgical and histopathological findings, the mucinous cystadenoma was regarded as primary retroperitoneal. This case demonstrates that in the era of radiological preoperative refinement, pathological diagnosis remains of utmost importance, especially for rare cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mucinous cystadenomas of the ovary are clinically and histopathologically well-established and common tumors. Primary retroperitoneal mucinous cystadenomas are extremely rare. Such tumors are histologically similar to ovarian mucinous cystadenomas. Their histogenesis is still unclear. We report a case of primary retroperitoneal mucinous cystadenoma with foci of borderline malignancy containing a mural “sarcoma-like” nodule.
Case report
Clinical history
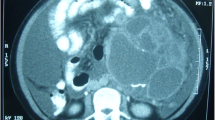
A 45-year-old, para 2, woman presented at the emergency room with a 3-week history of left-sided abdominal pain. She had felt a mass in the left lower quadrant 2 days before. Her clinical history included endometriosis and a car accident. The mass was progressive but not painful. Apart from the palpable mass of 15 cm in the left lower abdomen, physical examination was unremarkable. Ultrasonography demonstrated a 15-cm cystic mass with a 3.8-cm nodule in its wall. The uterus was normal in size, and internal ultrasonography showed small ovaries. Carcinoembryonic antigen, cancer antigen (CA) 125, and CA 19-9 levels were within normal limits. The next day, a contrast-enhanced computed tomography scan of the abdomen revealed a 15-cm left-sided cystic mass, which seemed to originate from the tail of the pancreas (Fig. 1a). The cystic mass showed a 4-cm nodule in its wall (Fig. 1b) and was suspected for a cystic papillary adenocarcinoma. At laparotomy, the cyst was not adhered to the pancreas and could be easily separated from its location near the tail without opening the pancreatic capsule. Vascularization appeared to arise from the mesentery of the left colon. It was localized in the retroperitoneal space extending caudally from the spleen to the lower abdomen with medial displacement of the left colon. Total resection of the cyst was performed, and the specimen was sent for histopathological examination. Further inspection showed two normal ovaries. Her postoperative recovery was uneventful. One year after surgery, the patient was without signs of recurrence or metastasis.
Materials and methods
The specimen was fixed in 4% buffered formalin. Representative samples were routinely processed and embedded in paraffin blocks. Four-micrometer-thick sections were stained with hematoxylin and eosin and with parallel routine immunohistochemical procedures. The antigens tested by immunohistochemistry were: pan-keratin, keratin Cam 5.2, cytokeratin 7, cytokeratin 10, cytokeratin 18, cytokeratin 20, epithelial membrane antigen, vimentin, desmin, actin, myosin, CD34, CD68, CD99, CD117, S-100 protein, and bcl-2.
Pathological findings
The specimen consisted of a unilocular cyst measuring 20 × 11 cm with a smooth surface. The content was a watery mucinous material. The wall was thin with a smooth gray-white inner surface and contained a circumscribed bean-shaped solid mural nodule of 3.5 × 3.5 × 2.5 cm, which showed a brown-yellow and focally hemorrhagic cut surface. Microscopically, most of the cyst was lined by single-layered tall columnar cells, abundant clear cytoplasm, and small basally located nuclei (Fig. 2a).
Cyst wall with typical tall columnar mucous-secreting epithelium and fibrous wall (a). Low cellular proliferation with some stratification of the cells and slight nuclear atypia (b). Heterogenous proliferation of pleomorphic cells, spindle cells, osteoclast-like giant cells, and some mononuclear cells with some pigment (c). Detail of sarcoma-like nodule (d)
Occasionally (over less than 1% of the surface), the epithelium showed slightly atypical proliferation with glandular budding, tufting of the epithelium, decreased cytoplasmic mucin, some stratification of slightly irregular nuclei, and an occasional mitoses (Fig. 2b). There was no infiltrative growth but foci of borderline malignancy. The nodule was well circumscribed without vascular invasion and consisted of a heterogenous population of spindle-shaped cells, pleomorphic cells with bizarre nuclei, mixed mononuclear inflammatory cells, benign osteoclast-like giant cells, and foci of hemorrhage. There were mitotic figures, including some atypical forms (Fig. 2c and d). The sarcoma-like cells proved to be keratin negative (Table 1).
Discussion
Mucinous cystadenomas can be located in the ovaries, pancreas, and in the retroperitoneum. The mucinous cystadenoma presented was localized retroperitoneally near the pancreas but was clearly not adhered to it. Because normal-appearing ovaries were found, the cystadenoma was thought to be primary retroperitoneal.
According to the literature, symptoms are nonspecific, and most patients complained of an abdominal distension or mass with or without pain [9]. Mucinous cystadenomas were relatively large, varying from 10 to greater than 20 cm in diameter, which is large enough to cause symptoms like abdominal fullness [9]. Preoperative diagnosis is very difficult, not because the tumors are often overlooked in the differential diagnosis but also because no sensitive methods or reliable markers are available [2]. As retroperitoneal mucinous cystadenomas are histologically similar to mucinous cystadenomas of the ovary, the ultrasonographic image pattern is in general of no help in distinguishing between ovarian and retroperitoneal origin. In our case, the diagnosis of the retroperitoneal mucinous cystadenoma could not be established preoperatively by ultrasonography or computed tomography. Although ultrasonography, computed tomography, or magnetic resonance imaging can detect retroperitoneal cysts, the diagnosis of mucinous cystadenoma is seldomly made preoperatively. The usual preoperative differential diagnosis consists of ovarian cyst, cystic mesothelioma, cystic lymphangioma, nonpancreatic pseudocyst, and renal cyst [4, 9, 18]. Although aspiration is a good method for delineating the nature of the cyst, cytologic analysis of the aspirated fluid frequently fails to reveal the type of epithelial cells lining the cyst. Therefore, exploratory laparotomy with complete excision of the cyst is usually indicated both for diagnosis and treatment [2].
Retroperitoneal mucinous cystadenomas are histologically similar to mucinous cystadenomas of the ovary. The histogenesis of these tumors is still unclear. Four main hypotheses have been proposed [2, 14]. According to the first three hypotheses, the tumor arises either from ectopic ovarian tissue, although ovarian tissue was only rarely found [14], from a teratoma in which the mucinous epithelium has overgrown all other components or from urogenital remnants. The most widely accepted theory suggests coelomic metaplasia as the causal agent, whereby tumors arise from invagination of the peritoneal mesothelial layer that undergoes mucinous metaplasia with cyst formation [3, 9]. Such origin rather than from ectopic ovarian tissue is supported by the occurrence of such a tumor in a male patients [5, 8, 10, 17].
Primary mucinous tumors of the retroperitoneum are very uncommon. These tumors can be classified into three clinicopathologic types: mucinous cystadenoma, mucinous cystic tumor of borderline malignancy, and mucinous cystadenocarcinoma. Our case was diagnosed as a primary retroperitoneal mucinous cystadenoma with only foci of borderline malignancy and a mural “sarcoma-like” nodule.
Mural nodules have been described in ovarian and pancreatic mucinous cystic tumors [7, 16]. Mural nodules may be malignant representing anaplastic carcinoma, containing a predominant population of cytokeratin-positive cells with high-grade malignant nuclei, or a genuine soft tissue-type sarcoma [7, 15, 16]. Benign pseudosarcomatous mural nodules are composed of a heterogeneous cell population of epulis-type giant cells, atypical spindle cells with bizarre nuclei and mitotic figures, mixed inflammatory cells, and signs of hemorrhage and necrosis. In these cases, immunohistochemical staining shows a weakly or focally cytokeratin positivity in the pseudosarcomatous cells.
We performed a literature review using Embase and Medline starting in 1966 and identified approximately 45 cases of retroperitoneal mucinous cystadenoma and 25 cases of mucinous cystadenocarcinoma. Only eight cases of mucinous cystadenoma with borderline malignancy have been reported (Table 2). Thus, our case is the ninth case of a retroperitoneal mucinous cystadenoma of borderline malignancy. By our knowledge, however, combination with a mural “sarcoma-like” nodule has not been described earlier in the literature. The patient should be followed. However, in this case, long-term follow-up seems not warranted regarding the only focal aspect (<1% of the surface) of borderline malignancy of the cyst and the benign reactive nature of the mural nodule.
References
Banerjee R, Gough J (1988) Cystic mucinous tumours of the mesentery and retroperitoneum: report of three cases. Histopathology 12:527–532
Chen JS, Lee WJ, Chang YJ, Wu MZ, Chiu KM (1998) Laparoscopic resection of a primary retroperitoneal mucinous cystadenoma: report of a case. Surg Today 28:343–345
Fujii S, Konishi I, Okamura H, Mori T (1986) Mucinous cystadenocarcinoma of the retroperitoneum: a light and electron microscopic study. Gynaecol Oncol 24:103–112
Ginsburg G, Fraser J, Saltzman B (1997) Retroperitoneal mucinous cystadenoma presenting as a renal cyst. J Urol 158:2232
Green JM, Bruner BC, Tang WW, Orihuela E (2007) Retroperitoneal mucinous cystadenocarcinoma in a man: a case report and review of the literature. Urol Oncol 25:53–55
Gutsu E, Mishin I, Gagauz I (2003) Primary retroperitoneal mucinous cystadenoma. A case report and brief review of the literature. Zentralbl Chir 128:691–693
Hamilton SR, Aaltonen LA (2000) Pathology and genetics of tumours of the digestive system. WHO Classification of Tumours. IARC, Lyon, p 236
Lai KKT, Chan YYR, Chin ACW, Ng WF, Huang YHH, Mak YLM, Wong WC (2004) Primary retroperitoneal mucinous cystadenoma in a 52-year-old man. J Hong Kong Coll Radiol 7:223–225
Matsubara M, Shiozawa T, Tachibana R, Hondo T, Osasda K, Kawaguchi K, Kimura K, Konishi I (2005) Primary retroperitoneal mucinous cystadenoma of borderline malignancy: a case report and review of the literature. Int J Gynaecol Pathol 24:218–223
Motoyama T, Chida T, Fujiwara T, Watanabe H (1994) Mucinous cystic tumor of the retroperitoneum. A report of two cases. Acta Cytol 38:261–266
Nagata J, Yamauchi M, Terabe K, Watanabe T, Ichihara H, Takagi H, Nakashima N (1987) A case of retroperitoneal mucinous cystadenoma of borderline malignancy. Nippon Geka Gakkai Zasshi 88:489–492
Papadogiannakis N, Gad A, Ehliar B (1997) Primary retroperitoneal mucinous tumor of low malignant potential: histogenetic aspects and review of the literature. APMIS Acta Pathol Microbiol Immunol Scand 105:483–486
Pearl ML, Valea F, Chumas J, Chalas E (1996) Primary retroperitoneal mucinous cystadenocarcinoma of low malignant potential: a case report and literature review. Gynecol Oncol 61:150–152
Pennel TC, Gusdon JP Jr (1989) Retroperitoneal mucinous cystadenoma. Am J Obstet Gynecol 160:1229–1231
Prat J (2004) Pathology of the ovary. Saunders, Philadelphia, pp 123–128
Tavassoli FA, Devilee P (2003) Pathology and genetics of tumours of the breast and female genital organs. WHO Classification of Tumours. IARC, Lyon, pp 127–129
Thamboo TP, Sim R, Tan SY, Yap WM (2006) Primary retroperitoneal mucinous cystadenocarcinoma in a male patient. J Clin Pathol 59:655–657
Yang DM, Jung DH, Kim H, Kang JH, Kim SH, Kim JH, Hwang HY (2004) Retroperitoneal cystic masses: CT, clinical, and pathological findings and literature review. Radiographics 24:1353–1365
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Article Précis
A 45-year-old woman complained of a left-sided abdominal mass, which turned out to be a primary retroperitoneal cystadenoma with mural nodule.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Bakker, R.F.R., Stoot, J.H.M.B., Blok, P. et al. Primary retroperitoneal mucinous cystadenoma with sarcoma-like mural nodule. Virchows Arch 451, 853–857 (2007). https://doi.org/10.1007/s00428-007-0479-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-007-0479-3