Abstract
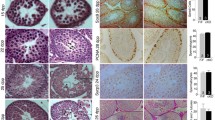
Testicular germ cell tumours (TGCT) exhibit remarkable ability to differentiate into virtually all somatic tissue types. In this study, we investigated changes in mucin-type O-glycosylation, which have been associated with somatic cell differentiation and cancer. Expression profile of simple mucin-type O-glycans (Tn, sialyl-Tn, T), histo-blood group H and A variants and six polypeptide GalNAc-transferases (T1–4, T6, T11) that control the site and density of O-glycosylation were analysed by immunohistochemistry during human testis development and in TGCT. Normal testis showed a restricted pattern; gonocytes expressed abundant sialyl-Tn and sialyl-T, and adult spermatogonia were devoid of any glycans, whereas spermatocytes and spermatids expressed exclusively glycans Tn and T and the GalNAc-T3 isoform. A subset of mature ejaculated spermatozoa expressed an additional glycan sialyl-T. The pattern found in testicular neoplasms recapitulated the developmental order: Pre-invasive carcinoma in situ (CIS) cells and seminoma expressed fetal type sialylated glycans in keeping with their gonocyte-like phenotype. Neither simple mucin-type O-glycans nor GalNAc-transferase isoforms were found in undifferentiated nonseminoma, i.e. embryonal carcinoma, whereas teratomas expressed them all to some extent but in a disorganized manner. We concluded that simple mucin-type O-glycans and their transferases are developmentally regulated in the human testis, with profound changes associated with neoplasia. The restricted O-glycosylation pattern in haploid germ cells suggests a role in their maturation or egg recognition/fertilization warranting further studies in male infertility, whereas the findings in TGCT provide new diagnostic tools and support our hypothesis that testicular cancer is a developmental disease of germ cell differentiation.




Similar content being viewed by others
References
Almstrup K, Hoei-Hansen CE, Wirkner U, Blake J, Schwager C, Ansorge W, Nielsen JE, Skakkebæk NE, Rajpert-De Meyts E, Leffers H (2004) Embryonic stem cell-like features of testicular carcinoma in situ revealed by genome-wide gene expression profiling. Cancer Res 64:4736–4743
Andrews PW (1984) Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol 103:285–293
Andrews PW, Banting GS, Damjanov I, Arnaud D, Avner P (1984) Three monoclonal antibodies defining distinct differentiation antigens associated with different high molecular weight polypetides on the surface of human embryonal carcinoma cells. Hybridoma 3:347–361
Andrews PW, Casper J, Damjanov I, Duggan-Keen M, Giwercman A, Hata J, von Keitz A, Looijenga LH, Millan JL, Oosterhuis JW, Pera M, Sawada M, Schmoll HJ, Skakkebaek NE, van Putten W, Stern P (1996) Comparative analysis of cell surface antigens expressed by cell lines derived from human germ cell tumours. Int J Cancer 66:806–816
Bailey D, Baumal R, Law J, Sheldon K, Kannampuzha P, Stratis M, Kahn H, Marks A (1986) Production of a monoclonal antibody specific for seminomas and dysgerminomas. Proc Natl Acad Sci U S A 83:5291–5295
Bennett EP, Hassan H, Mandel U, Mirgorodskaya E, Roepstorff P, Burchell J, Taylor-Papadimitriou T, Hollingsworth MA, Merkx G, van Kessel AG, Eiberg H, Steffensen R, Clausen H (1998) Cloning of a human UDP-N-acetyl-. α. -d-galactosamine: polypeptide N-acetylgalactosaminyltransferase that complements other GalNAc-transferases in complete O-glycosylation of the MUC1 tandem repeat. J Biol Chem 273:30472–30481
Bennett EP, Weghuis DO, Merkx G, van Kessel AG, Eiberg H, Clausen H (1998) Genomic organisation and chromosomal localisation of three members of the UDP-N-acetylgalactosamine: polypetide N-acetylgalactosaminyltransferase family. Glycobiology 8:547–555
Bennett EP, Hassan H, Mandel U, Hollingsworth MA, Akisawa N, Ikematsu Y, Merkx G, van Kessel AG, Olofsson S, Clausen H (1999) Cloning and characterization of a close homologue of human UDP-N-acetyl-alpha-d-galactosamine: polypeptide N-acetylgalactosaminyl-transferase-T3, designated GalNAc-T6. Evidence for genetic but not functional redundancy. J Biol Chem 274:25362–25370
Brockhausen I (2006) Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Reports 7:599–604
Campagnoli MF, Pucci A, Garelli E, Carando A, Defilippi C, Lala R, Ingrosso G, Dianzani I, Forni M, Ramenghi U (2006) Familial tumoral calcinosis and testicular microlithiasis associated with a new mutation of GALNT3 in a white family. J Clin Pathol 59:440–442
Chen J, Litscher ES, Wassarman PM (1998) Inactivation of the mouse sperm receptor, mZP3, by site-directed mutagenesis of individual serine residues located at the combining site for sperm. Proc Natl Acad Sci U S A 95:6193–6197
Clausen H, Bennett EP (1996) A family of UDP-GalNAc: polypeptide N-acetylgalactosaminyl-transferases control the initiation of mucin-type O-linked glycosylation. Glycobiology 6:635–646
Dabelsteen E, Jacobsen GK (1991) Histo-blood group antigens as differentiation markers in testicular germ cell tumours. APMIS 99:391–397
Dube DH, Bertozzi CR (2005) Glycans in cancer and inflammation—potential for therapeutics and diagnostics. Nat Rev Drug Discov 4:477–488
Ensslin MA, Shur BD (2003) Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin-domain protein involved in sperm–egg binding. Cell 114:405–417
Fuster MM, Esko FD (2005) The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer 5:526–542
Hardy DM, Garbers DL (1995) A sperm membrane protein that binds in a species-specific manner to the egg extracellular matrix is homologous to von Willebrand factor. J Biol Chem 270:26025–26028
Hart AH, Hartley L, Parker K, Ibrahim M, Looijenga LH, Pauchnik M, Chow CW, Robb L (2005) The pluripotency homeobox gene NANOG is expressed in human germ cell tumors. Cancer 104:2092–2098
Hassan H, Bennett EP, Mandel U, Hollingsworth MA, Clausen H (2000) Control of mucin-type O-glycosylation: O-glycan occupancy is directed by substrate specificities of polypeptide GalNAc-transferases. In: Ernst, Hart, Sinay (eds) Carbohydrates in chemistry and biology. A comprehension handbook. Wiley, New York, pp 273–292
Hoei-Hansen CE, Almstrup K, Nielsen JE, Brask Sonne S, Graem N, Skakkebaek NE, Leffers H, Rajpert-De Meyts E (2005) Stem cell pluripotency factor NANOG is expressed in human fetal gonocytes, testicular carcinoma in situ and germ cell tumours. Histopathology 47:48–56
Ichikawa S, Lyles KW, Econs MJ (2005) A novel GALNT3 mutation in a pseudoautosomal dominant form of tumoral calcinosis: evidence that the disorder is autosomal recessive. J Clin Endocrinol Metab 90:2420–2423
Jacobsen GK, Nørgaard-Pedersen B (1984) Placental alkaline phosphatase in testicular germ cell tumours and carcinoma in situ of the testis. APMIS A 92:323–329
Jørgensen N, Rajpert-De Meyts E, Græm N, Müller J, Giwercman A, Skakkebæk NE (1995) Expression of immunohistochemical markers for testicular carcinoma-in situ by normal human fetal germ cells. Lab Invest 72:223–231
Jørgensen N, Carlsen E, Nermoen I, Punab M, Suominen J, Andersen A-G, Andersson A-M, Haugen TB, Horte A, Jensen TK, Magnus Ø, Petersen JH, Vierula M, Toppari J, Skakkebæk NE (2002) East–West gradient in semen quality in the Nordic-Baltic area: A study of men from the general population in Denmark, Norway, Estonia and Finland. Hum Reprod 17:2199–2208
Julien S, Krzewinski-Recchi MA, Harduin-Lepers A, Gouyer V, Huet G, Le Bourhis X, Delannoy P (2001) Expression of sialyl-Tn antigen in breast cancer cells transfected with the human CMP-Neu5Ac:GalNAc alpha2,6-sialyltransferase (ST6GalNAc I) cDNA. Glycoconj J 18:883–893
Kaneko M, Kato Y, Kunita A, Fujita N, Tsuruo T, Osawa M (2004) Functional sialylated O-glycan to platelet aggregation on Aggrus (T1alpha/Podoplanin) molecules expressed in Chinese hamster ovary cells. J Biol Chem 279:38838–38843
Kang JL, Rajpert-De Meyts E, Wiels J, Skakkebaek NE (1995) Expression of the glycolipid globo-triaosylceramide (Gb3) in testicular carcinoma in situ. Virchows Arch 426:369–374
Looijenga LHJ, Stoop H, De Leeuw PJC, De Gouveia Brazao CA, Gillis AJM, van Roozendaal KE, van Zoelen EJ, Weber RF, Wolffenbuttel KP, van Dekken H, Honecker F, Bokemeyer C, Perlman EJ, Schneider DT, Kononen J, Sauter G, Oosterhuis JW (2003) POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res 63:2244–2250
Mandel U, Petersen OW, Sørensen H, Vedtofte P, Hakomori SI, Clausen H, Dabelsteen E (1991) Simple mucin-type carbohydrates in oral stratified squamous and salivary gland epithelia. J Invest Dermatol 97:713–721
Mandel U, Hassan H, Therkildsen H, Rygaard J, Jacobsen MH, Juhl BR, Dabelsteen E, Clausen H (1999) Expression of polypeptide GalNAc-transferases in stratified epithelia and squamous cell carcinomas: immunohistological evaluation using monoclonal antibodies to three members of the GalNAc-transferase family. Glycobiology 9:43–52
Olie RA, Fenderson B, Daley K, Oosterhuis JW, Murphy J, Looijenga LH (1996) Glycolipids of human primary testicular germ cell tumours. Br J Cancer 74:133–140
Rajpert-De Meyts E, Jørgensen NE, Nielsen KB, Müller J, Skakkebæk NE (1998) Developmental arrest of germ cells in the pathogenesis of germ cell neoplasia. APMIS 106:198–206
Rajpert-De Meyts E, Bartkova J, Samson M, Hoei-Hansen CE, Frydelund-Larsen L, Bartek J, Skakkebæk NE (2003) The emerging phenotype of the testicular carcinoma in situ cell. APMIS 111:267–279
Rajpert-De Meyts E (2006) Developmental model for the pathogenesis of testicular carcinoma in situ: environmental and genetic aspects. Hum Reprod Updat 12:303–323
Sandhoff R, Geyer R, Jennemann R, Paret C, Kiss E, Yamashita T, Gorgas K, Sijmonsma TP, Iwamori M, Finaz C, Proia RL, Wiegandt H, Gröne HJ (2005) Novel class of glycosphingolipids involved in male fertility. J Biol Chem 29:27310–27318
Schacht V, Dadras SS, Johnson LA, Jackson DG, Hong YK, Detmar M (2005) Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol 166:913–921
Schwientek TJ, Bennett EP, Flores C, Thacker J, Hollman M, Reis CA, Behrens J, Mandel U, Keck B, Schafer MA, Hazelmann K, Zubarev R, Roepstorff P, Hollingsworth MA, Clausen H (2002) Functional conservation of subfamilies of putative UDP-N-acetylgalactosamine: polypeptide N-acetylgalactosaminyltransferases in drosophila, C. elegans and mammals: one subfamily comprised of l(2)35Aa is essential in drosophila. J Biol Chem 277:22623–22638
Skakkebæk NE (1972) Possible carcinoma-in-situ of the testis. Lancet 2:516–517
Skakkebæk NE, Berthelsen JG, Giwercman A, Müller J (1987) Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl 10:19–28
Sonne SB, Herlihy AS, Hoei-Hansen C, Nielsen JE, Almstrup K, Skakkebæk NE, Marks A, Leffers H, Rajpert-De Meyts E (2006) Identity of M2A (D2–40) antigen and gp36 (Aggrus, T1-A2, podoplanin) in human developing testis, testicular carcinoma in situ and germ-cell tumours. Virchows Archiv 449:200–206
Springer GF (1984) T and Tn, general carcinoma autoantigens. Science 224:1198–1206
Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, Khamaysi Z, Behar D, Petronius D, Friedman V, Zelikovic I, Raimer S, Metzker A, Richard G, Sprecher E (2004) Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet 36:579–581
Topaz O, Bergman R, Mandel U, Maor G, Goldberg R, Richard G, Sprecher E (2005) Absence of intraepidermal glycosyltransferase ppGalNac-T3 expression in familial tumoral calcinosis. Am J Dermatopathol 27:211–215
Töpfer-Petersen E (1999) Carbohydrate-based interactions on the route of a spermatozoon to fertilization. Hum Reprod Updat 5:314–329
Wenk J, Andrews PW, Casper J, Hata J, Pera MF, von Keitz A, Damjanov I, Fenderson BA (1994) Glycolipids of germ cell tumors: extended globo-series glycolipids are a hallmark of human embryonal carcinoma cells. Int J Cancer 58:108–115
Acknowledgement
The authors thank Professor P. W. Andrews for a generous gift of NT/D1 cell line and TRA-1-60 antibody, Dr N. Jørgensen for fetal tissue specimens, the pathologists at several pathology departments in the Greater Copenhagen area for assistance in obtaining tissue specimens and L. Andersen, H. Kistrup and T. Adelfest for skilful technical assistance. This work was supported by grants from the Danish Cancer Society, the Svend Andersen Foundation, the Vissing Foundation, the Kirsten and Freddy Johansen Foundation, the Willumsen Foundation and the Danish Medical Research Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
E. Rajpert-De Meyts and S. N. Poll contributed equally to this study.
Rights and permissions
About this article
Cite this article
Rajpert-De Meyts, E., Poll, S.N., Goukasian, I. et al. Changes in the profile of simple mucin-type O-glycans and polypeptide GalNAc-transferases in human testis and testicular neoplasms are associated with germ cell maturation and tumour differentiation. Virchows Arch 451, 805–814 (2007). https://doi.org/10.1007/s00428-007-0478-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-007-0478-4