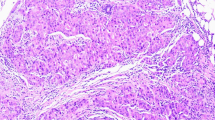
Abstract
Epithelial cells of fetal breast glandular structures, at the third trimester of pregnancy (28 weeks), produce GCDFP-15, in the absence of specific apocrine morphology. Apocrine epithelium of the breast may be a normal process of differentiation rather than a result of metaplasia, and it has been demonstrated that it is estrogen-receptor, progesterone-receptor and bcl-2 negative, but androgen-receptor (AR) positive. The significance of AR expression in apocrine epithelium is uncertain. Apocrine epithelium is seen in a wide spectrum of breast entities, ranging from benign lesions to invasive carcinoma. Breast cancer accounts 32% of all cancer cases among women and is the most common type of cancer in women. Little is known about breast carcinogenesis. Widely, it is accepted that breast cancer, like most other type of cancer, is being developed through the accumulation of genetic aberrations. Apocrine epithelium may reflect instability of the breast epithelium, creating an environment favouring further oncogenic alterations. In the last decade, several lines of evidence support the idea that some breast benign epithelial apocrine lesions are clonal lesions and may be considered as truly pre-malignant or precursors of breast carcinoma. Apocrine changes in many cases do not present any diagnostic difficulty; on the other hand, apocrine proliferations with cytologic atypia can be particularly difficult and challenging. The purpose of this study is to collect and highlight the areas of consensus in the literature as well as the controversial areas concerning the apocrine epithelium of the breast.






Similar content being viewed by others
References
Abati AD, Kimmel M, Rosen PP (1990) Apocrine mammary carcinoma. A clinicopathologic study of 72 cases. Am J Clin Pathol 94(4):371–377
Agnantis NJ, Mahera H, Maounis N, Spandidos DA (1992) Immunohistochemical study of ras and myc oncoproteins in apocrine breast lesions with and without papillomatosis. Eur J Gynaecol Oncol 13:309–331
Ahmed A (1975) Apocrine metaplasia in cystic hyperplastic mastopathy. Histochemical and ultrastructural observations. Pathol (Lond) 115:211–214
Azzopardi J (1979) Problems in breast pathology. Saunders, Philadelphia, pp 341–344
Baddoura FK, Judd RL (1990) Apocrine adenoma of the breast. Report of a case with investigation of lectin binding patterns in apocrine breast lesions. Mod Pathol 3:373–376
Belanger A, Caron S, Labrie F et al (1990) Levels of eighteen nonconjugated and conjugated steroids in human breast cysts fluid; relationship with cyst type. Eur J Cancer 26:277–281
Boccardo F, Valenti G, Zanardi S, Cerruti G, Fassio T, Bruzzi P, De Francis V, Barreca A, Del Monte P, Minuto F (1988) Epidermal growth factor in breast cyst fluid: relationship with intracystic cation and androgen conjugate content. Cancer Res 45:5860–5863
Bruzzi P, Dogliotti L, Naldoni C et al (1997) Cohort study of association risk of breast cancer. BMJ 314:925–928
Bryant J (1981) Male breast cancer: a case of apocrine carcinoma with psammoma bodies. Hum Pathol 12:751–753
Bussolati G, Sapino A, Gugliotta P, Marci L (1992) Cytological analysis of benign breast disease. Cancer Detect Prev 16:89–92
Bundred NJ, West RR, O'Dowd J, Mansel RE (1991) Is there an increased risk of breast cancer in women who have a breast cyst aspirated? Br J Cancer 64:953–955
Cancer Committee of the College of American Pathologists (1986) Is ‘fibrocystic disease’ of the breast precancerous? Arch Pathol Lab Med 110:171–173
Carter DJ, Rosen PP (1991) Atypical apocrine metaplasia in sclerosing lesions of the breast: a study of 51 patients. Mod Pathol 4:1–5
Castagnetta L, Granata OM, Brignone G, Blasi L, Arcuri F, Mesiti MD, Aquino A, Preitano W (1990) Steroid patterns of benign breast disease. Ann N Y Acad Sci 586:79–82
Collett K, Maehle BO, Skjarven R et al (1996) Prognostic role of oestrogen, progesterone and androgen receptor in relation to patient age in patient age in patients with breast cancer. Breast 5:123–126
Collette J, Hendrick JC, Jaspar JM, Franchimont P (1986) Presence of alpha-lactalbumin, epidermal growth factor, epithelial membrane antigen, and gross cystic disease fluid protein (15,000 Daltons) in breast cyst fluid. Cancer Res 46(7):3728–3733
Constantini M, Bucchi I, Dogliotti et al (1994) Cohort study of women with aspirated gross cysts of the breast: an update in recent developments in the study of benign breast disease. In: Mansel RE (ed) Recent developments in the study of benign breast disease. Parthenon Publishing Group, Manchester, pp 227–240
Cotran RS, Kumar V, Robbins SL (1994) Cellular growth and differentiation. In: Robbins SL (ed) Robbins pathologic basis of disease, 5th edn. WB Saunders, Philadelphia, pp 35–50
Dawson EK (1932) Sweat gland carcinoma of the breast. A morphohistological study. Edinb Med J 39:409–438
Dixon JM, McDonald, Elton RA, Miller WR (1999) Risk of breast cancer in women with palpable breast cysts: a prospective study. Edinburgh Breast Group. Lancet 353:1742–1745
Duggan MA, Young GK, Hwang WS (1998) Fine-needle aspiration of an apocrine breast carcinoma with multivacuolated lipid-rich giant cells. Diagn Cytopathol 4:62–66
Dupont WD, Page DL (1985) Risk factors for breast cancer in women with proliferative disease. N Engl J Med 312:146–151
Durham JR, Fechner RE (2000) The histologic spectrum of apocrine lesions of the breast. Am J Clin Pathol 113:3–18
Ellis LM, Wittliff L, Bryant MS et al (1989) Correlation of oestrogen, progesterone and androgen receptors in breast cancer. Am J Surg 157:577–581
Eusebi V, Azzopardi JG (1980) Lobular endocrine neoplasia in fibroadenoma of the breast. Histopathology 4:413–428
Eusebi V, Betts CM, Haagensen DE, Bussolati G, Azzopardi JG (1984) Apocrine differentiation in lobular carcinoma of the breast. Hum Pathol 15:134–140
Eusebi V, Damiani S, Losi L, Millis RR (1997) Apocrine differentiation in breast epithelium. Adv Anat Pathol 4:139–155
Frable WJ (1989) Needle aspiration biopsy: past, present, and future. Hum Pathol 20:504–517
Frable WJ, Kay S (1968) Carcinoma of the breast. Histologic and clinical features of apocrine tumors. Cancer 21:756–763
Gatalica Z (1997) Immunohistochemical analyses of apocrine breast lesions. Consistent over-expression of androgen receptor accompanied by the loss of estrogen and progesterone receptors in apocrine metaplasia and apocrine carcinoma in situ. Pathol Res Pract 193:753–758
Gilles R, Guinebretiere JM, Toussaint C et al (1994) Locally advanced breast cancer: contrast-enhanced subtraction MR imaging of response to preoperative chemotherapy. Radiology 191:633–638
Gupta RK, Wakefield SJ, Naran S, Dowle CC (1989) Immunocytochemical and ultra-structural diagnosis of a rare mixed apocrine-medullary carcinoma of the breast in a fine needle aspirate. Acta Cytol 33:104–108
Haagensen DE Jr, Mazoujian G (1996) In: Haagenson, CD (ed) Diseases of the breast, 3rd edn. WB Saunders, Philadelphia, pp 474–500
Haagensen DE Jr, Gall SA, Brazy JE, Giannola J, Wells SA Jr (1980) Analysis of amniotic fluid, maternal plasma, and cord blood for a human breast gross cystic disease fluid protein. Am J Obstet Gynecol 138(1):25–32
Haagensen CD, Bodian C, Haagensen DE Jr (1981) Apocrine epithelium. Breast carcinoma, WB Saunders, Philadelphia pp 83–105
Haagensen DE, Dilley WG, Mazoujian G et al (1990) Review of GCDFP-15: an apocrine marker protein. Ann N Y Acad Sci 586:161–173
Higginson JF, McDonald JR (1949) Apocrine tissue, chronic cystic mastitis and sweat gland carcinoma of the breast. Surg Gynecol Obstet 88:1–10
Howard BA, Gusterson BA (2000) Human breast development. J Mammary Gland Biol Neoplasia 5(2):119–137
Izuo M, Okagaki T, Richart R, Lattes R (1971) DNA content in ‘apocrine metaplasia’of fibrocystic disease of the breast. Cancer 27:643–650
Jones C, Damiani S, Wells D, Chaggar R, Lakhani SR, Eusebi V (2001) Molecular cytogenetic comparison of apocrine hyperplasia and apocrine carcinoma of the breast. Am J Pathol 158:207–214
Kaya H, Aribal E, Yegen C (2002) Apocrine differentiation in invasive pleomorphic lobular carcinoma with in situ ductal and lobular apocrine carcinoma: case report. Pathol Oncol Res 8(2):151–152
Kline TS (1988) Handbook of fine needle aspiration biopsy cytology. CV Mosby, St. Louis
Kuenen-Boumeester V, Van der Kwast TH, van Putten WL, Claasen C, van Ooijen B, Henzen-Longmans SC (1992) Immunohistochemical determination of androgen receptors in relation to oestrogen and progesterone receptors in female breast cancer. Int J Cancer 52:581–584
Lakhani SR, Slack DN, Hamoudi RAA, Collins N, Stratton MR, Sloane JP (1996) Detection of allelic imbalance indicates that a proportion of mammary hyperplasia of usual type is clonal, neoplastic proliferations. Lab Invest 74:129–135
Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D et al (2001) Initial sequencing and analysis of the human genome. Nature 409:860–921
Lininger RA, Tavassoli FA (1997) Atypical intraductal hyperplasia of the breast. In: Silverstein MJ (ed) Ductal carcinoma in situ of the breast, Williams & Wilkins, Baltimore, pp 195–222
Lodato RF, Maguire HC Jr, Greene MI, Weiner DB, LiVolsi VA (1990) Immunohistochemical evaluation of c-erbB-2 oncogene expression in ductal carcinoma in situ and atypical ductal hyperplasia of the breast. Mod Pathol 3(4):449–454
Losi L, Lorenzini R, Eusebi V, Bussolati G (1995) Apocrine differentiation in invasive carcinoma of the breast. Comparison of monoclonal and polyclonal gross cystic disease fluid protein-15 antibodies with prolactin-inducible protein mRNA gene expression. Appl Immunohistochem 3:91–98
Love SM, Gelman RS, Silen W (1982 Oct 14) Sounding board. Fibrocystic Disease of the breast—a non disease? N Engl J Med 307(16):1010–1014
Matsuo K, Fukutomi T, Hasegawa T, Akashi-Tanaka S, Nanasawa T, Tsuda H (2002) Histological and immunohistochemical analysis of apocrine breast carcinoma. Breast Cancer 9:43–49
McKiernan O, Coyne J, Cahalane S (1988) Histology of breast development in early life. Arch Dis Child 63:136–139
Miller WR, Forrest APM (1983) Androgen conjugates in human breast secretions and cyst fluids. In: Angeli A, Bradlow HL, Dogliotti L (eds) Endocrinology of cystic disease. Raven Press, New York, pp 77–84
Moriya T, Sakamoto K, Sasano H, Kawanaka M, Sonoo H, Manabe T, Ito J (2000) Immunohistochemical analysis of Ki-67, p53, p21 and p27 in benign and malignant apocrine lesions of the breast: its correlation to histologic findings in 43 cases. Mod Pathol 13:13–18
Mossler JA, Barton TK, Brinkhous AD et al (2005) Apocrine differentiation in human mammary carcinoma. Cancer 46:2463–2471
Moinfar F, Okcu M, Tsybrovskyy O et al (2003) Androgen receptors frequently are expressed in breast carcinomas: potential relevance to new therapeutic strategies. Cancer 9:335–336
Murad TM, von Hamm E (1968) The ultrastructure of fibrocystic disease of the breast. Cancer 22:587–600
Murphy LC, Lee-Wing M, Goldenberg GJ, Shiu RPC (1987) Cancer Res 47:4160–4164
O'Malley FP, Page DL, Nelson EH, Dupont WD (1994) Ductal carcinoma in situ of the breast with apocrine cytology: definition of a borderline category. Hum Pathol 25:164–168
Pagani A, Sapino A, Eusebi V, Bergnolo P, Bussolati G (1994) PIP/GCDFP-15 gene expression and apocrine differentiation in carcinomas of the breast. Virchows Arch 425:459–465
Page DL, Van der Zwaar R, Rogers LW, Williams LT, Walker WE, Hartmann WH (1978) Relation between component parts of fibrocystic disease complex and breast cancer. J Natl Cancer Inst 61:1055–1063
Page DL, Dupont WD, Jensen RA (1996) Papillary apocrine change of the breast: association with atypical hyperplasia and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 5:29–32
Papamichalis G, Francia K, Karachaliou FE, Anastasiades OT, Spandidos DA (1988) Expression of the c-myc oncoprotein in human metaplastic epithelial cells of fibrocystic disease. Anticancer Res 8:1217–1222
Papanicolaou GN (1963) Atlas of exfoliative cytology. Harvard University Press, Cambridge, p 55
Papotti M, Eusebi V, Gugliotta P et al (1983) Immunohistochemical analysis of benign and malignant papillary lesions of the breast. Am J Surg Pathol 7:451–461
Pier WJ, Garancis JC, Kuzma JF (1970) The ultrastructure of apocrine cells in intracystic papilloma and fibrocystic disease of the breast. Arch Pathol 89:446–452
Raju U, Zarbo RJ, Kubus J et al (1993) The histologic spectrum of apocrine breast proliferations: a comparative study of morphology and DNA content by image analysis. Hum Pathol 24:173–181
Rosai J (1996) Ackerman's surgical pathology, 8th edn. CV Mosby, St. Louis, pp1582–1583
Roberts MM, Jones V, Elton RA et al (1984) Risk of breast cancer in women with a history of benign disease of the breast. BMJ 288:275–278
Rosen PP (2001) Rosen's Breast Pathology, 2nd edn. Lippincott Williams & Wilkins, Philadelphia
Russo J, Russo IH (2004) Development of the human breast. Maturitas 24;49(1):2–15
Sapp M, Malik A, Hanna W (2003) Hormone receptor profile of apocrine lesions of the breast. Breast J 9:335–336
Selim AG, Wells CA (1999) Immunohistochemical localization of androgen receptor in apocrine metaplasia and apocrine adenosis of the breast: relation to oestrogen and progesterone receptors. J Clin Pathol 52:838–841
Selim AG, Ryan A, El-Ayat G, Wells CA (2001) Loss of heterozygosity and allelic imbalance in apocrine adenosis of the breast. Cancer Detect Prev 25:262–267
Selim AGA, El-Ayat, Wells CA (2002) Expression of c-erbB2, p53, Bcl-2, Bax, c-myc and Ki-67 in apocrine metaplasia and apocrine change within sclerosing adenosis of the breast. Virchows Arch 441:449–455
Selim AG, Ryan A, El-Ayat G, Wells CA (2002) Loss of heterozygosity and allelic imbalance in apocrine metaplasia of the breast: microdissection microsatellite analysis. J Pathol 196:287–291
Seidman JD, Ashton M, Lefkowitz M (1996) Atypical apocrine adenosis of the breast: a clinicopathologic study of 37 patients with 8.7-year follow up. Cancer 77:2529–2537
Shiu RPC, Iwasiow BM (1985) Prolactin inducible proteins in human breast cancer cells. J Biol Chem 260:11307–11313
Silverberg SG, Masood S (1997) The breast. In: Silverberg SG, Delellis RA, Frable WJ (eds) Principles and practice of surgical pathology and cytopathology, 3rd edn. Churchill Livingstone, New York, pp 575–674
Simpson JF, Page DL, Dupont WD (1990) Apocrine adenosis—a mimic of mammary carcinoma. Surg Pathol 3:289–299
Simpson PT, Reis-Fihlo JS, Gale T, Lakhani S (2005) Molecular evolution of breast cancer. J Pathol 205:248–254
Sternberg SS (1997) Histology for pathologists, 2nd edn. Lippincott-Raven, Philadelphia, p 26
Tashiro T, Hirokawa M, Iuchi K, Emoto K, Tanaka T, Monobe Y, Sano T (2003) Cytology of pleomorphic lobular carcinoma with apocrine cell differentiation of the breast. A case report. Acta Cytol 47(2):265–269
Tavassoli FA, Norris HJ (1994) Intraductal apocrine carcinoma: a clinicopathologic study of 37 cases. Mod Pathol 7:813–818
Tavassoli F, Devilee P (2003) Pathology and genetics of tumors of the breast and female genital organs. IARC Press, Lyon, p 10
The Consensus Conference Committee (1997) Consensus conference on the classification of ductal carcinoma in situ. Cancer 80:1798–1802
Tremblay G (1968) Histochemical studies of oxidative enzymes in apocrine like cells of the breast and in axillary apocrine glands. J Invest Dermatol 50:238–243
Tsuda H, Fukutomi T, Hirohashi S (1995) Pattern of gene alterations in intraductal breast neoplasms associated with histological type and grade. Clin Cancer Res 1:261–267
Vasiu R, Olinici CD (1990) DNA nuclear content of apocrine cells in fibrocystic mastopathy and breast cancer. Morphol Embryol 36:43–47
Viacava P, Naccarato AG, Bevilaqua G (1997) Apocrine epithelium of the breast: does it result from metaplasia? Virchows Arch 431:205–2090
Washington C, Dalbegue F, Abreo F, Taubenberger JK, Lichy JH (2000) Loss of heterozygosity in fibrocystic change of the breast: genetic relationship between benign proliferative lesions and associated carcinomas. Am J Pathol 157:323–329
Wellings SR, Alpers CE (1987) Apocrine cystic metaplasia: subgross pathology and prevalence in cancer-associated versus random autopsy breasts. Hum Pathol 18:381–386
Wellings SR, Jensen HM, Marcum RG (1975) An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst 55:231–243
Wells CA, McGregor IL, Makunura CN, Yeomans P, Davies JD (1995) Apocrine adenosis: a precursor of aggressive breast cancer? J Clin Pathol 48:737–742
Wick MR, Lillemoe TJ, Copland GT, Swanson PE, Manivel JC, Kiang DT (1989) Gross cystic disease fluid protein-15 as a marker for breast cancer: immunohistochemical analysis of 690 human neoplasms and comparison with a-lactalbumin. Hum Pathol 20:281–287
Yoshida K, Inone M, Furuta S, Sakai R, Imai R, Hayakawa S, Fukatsu T, Nagasaka T, Nakashima N (1996) Apocrine carcinoma versus Apocrine metaplasia with atypia of the breast. Use of aspiration biopsy cytology. Acta Cytol 40(2):247–251
Zajdela A, Ghossein NA, Pilleran JP et al (1975) The experience of aspiration cytology in the diagnosis of breast cancer. Experience at the Fondation Curie. Cancer 35:499–506
Zoran G (1997) Immunohistochemical analysis of apocrine breast lesions: consistent overexpression of androgen receptor accompanied by the loss of estrogen and progesterone receptors in apocrine metaplasia and apocrine in situ. Pathol Res Pract 193:753–758
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zagorianakou, P., Zagorianakou, N., Stefanou, D. et al. The enigmatic nature of apocrine breast lesions. Virchows Arch 448, 525–531 (2006). https://doi.org/10.1007/s00428-005-0095-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-005-0095-z