Abstract
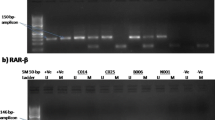
The newly identified 3p21.3 tumor suppressor gene RASSF1A is inactivated by hypermethylation in variable solid tumors, including those of the lung, breast, prostate, kidney, and ovary. The purpose of this study was to evaluate the methylation status of RASSF1A in various types and stages of ovarian epithelial tumors. We analyzed the DNA methylation status of ovarian tumors using methylation-specific polymerase chain reaction in 54 frozen ovarian tumor tissues and in 97 cases of archival ovarian serous epithelial tumors using a microdissection procedure. Hypermethylation statuses were examined vs clinicopathologic findings. RASSF1A promoter methylation rates in the various types of fresh ovarian tissues were as follows: serous cystadenoma (1/5), serous tumor of borderline malignancy (2/7), serous adenocarcinoma (4/10), mucinous cystadenoma (0/5), mucinous tumor of borderline malignancy (2/7), mucinous adenocarcinoma (3/6), transitional-cell carcinoma (1/3), clear-cell carcinoma (3/3), and malignant müllerian mixed tumor (3/3). In archived serous tumor tissues, RASSF1A promoter hypermethylation was detected in serous cystadenoma (1/6, 16.6%), serous tumor of borderline malignancy (20/41, 48.8%), and in serous adenocarcinoma (25/50, 50%). The status of RASSF1A hypermethylation in borderline tumors was found to correlate statistically with the presence of microinvasion (p=0.002), peritoneal implant (p<0.001), and bilaterality (p=0.019). The RASSF1A promoter hypermethylation was frequently found in borderline tumors and carcinomas, suggesting that RASSF1A promoter hypermethylation may be a useful molecular marker for the early detection of ovarian tumors.

Similar content being viewed by others
References
Agathanggelou A, Honorio S, Macartney DP, Martinez A, Dallol A, Rader J, Fullwood P, Chauhan A, Walker R, Shaw JA, Hosoe S, Lerman MI, Minna JD, Maher ER, Latif F (2001) Methylation associated inactivation of RASSF1A from region 3p21.3 in lung, breast and ovarian tumours. Oncogene 20:1509–1518
Bell DA, Weinstock MA, Scully RE (1988) Peritoneal implants of ovarian serous borderline tumors. Histologic features and prognosis. Cancer 62:2212–2222
Bell KA, Smith Sehdev AE, Kurman RJ (2001) Refined diagnostic criteria for implants associated with ovarian atypical proliferative serous tumors (borderline) and micropapillary serous carcinomas. Am J Surg Pathol 25:419–432
Dammann R, Yang G, Pfeifer PF (2001) Hypermethylation of the CpG island of Ras association domain family 1A (RASSF1A), a putative tumor suppressor gene from the 3p21.3 locus, occurs in a large percentage of human breast cancers. Cancer Res 61:3105–3109
de Nictolis M, Montironi R, Tommasoni S, Carinelli S, Ojeda B, Matias-Guiu X, Prat J (1992) Serous borderline tumors of the ovary. A clinicopathologic, immunohistochemical, and quantitative study of 44 cases. Cancer 70:152–160
Ford D, Easton D (1995) The genetics of breast and ovarian cancer. Br J Cancer 72:805–812
Fullwood P, Marchini S, Rader JS, Martinez A, Macartney D, Broggini M, Morelli C, Barbanti-Brodano G, Maher ER, Latif F (1999) Detailed genetic and physical mapping of tumor suppressor loci on chromosome 3p in ovarian cancer. Cancer Res 59:4662–4667
Honorio S, Agathanggelou A, Schuermann M, Pankow W, Viacava P, Maher ER, Latif F (2003) Detection of RASSF1A aberrant promoter hypermethylation in sputum from chronic smokers and ductal carcinoma in situ from breast cancer patients. Oncogene 22:147–150
Ibanez de Caceres I, Battagli C, Esteller M, Herman JG, Dulaimi E, Edelson MI, Bergman C, Ehya H, Eisenberg BL, Cairns P (2004) Tumor cell-specific BRCA1 and RASSF1A hypermethylation in serum, plasma, and peritoneal fluid from ovarian cancer patients. Cancer Res 64:6476–6481
Lee JY, Dong SM, Kim SY, Yoo NJ, Lee SH, Park WS (1998) A simple, precise and economical microdissection technique for analysis of genomic DNA from archival tissue sections. Virchows Arch 433:305–309
Lee MG, Kim HY, Byun DS, Lee SJ, Lee CH, Kim JI, Chang SG, Chi SG (2001) Frequent epigenetic inactivation of RASSF1A in human bladder carcinoma. Cancer Res 61:6688–6692
Murdoch WJ (1996) Ovarian surface epithelium, ovulation and carcinogenesis. Biol Rev Camb Philos Soc 71:529–543
NIH consensus conference (1995) Ovarian cancer. Screening, treatment and follow-up. NIH Consensus Development Panel on Ovarian Cancer. JAMA 273:491–497
Rathi A, Virmani AK, Schorge JO, Elias KJ, Maruyama R, Minna JD, Mok SC, Girard L, Fishman DA, Gazdar AF (2002) Methylation profiles of sporadic ovarian tumors and nonmalignant ovaries from high-risk women. Clin Cancer Res 8:3324–3331
Rimessi P, Gualandi F, Morelli C, Trabanell C, Wu Q, Possati L, Montesi M, Barrett JC, Barbanti-Brodano G (1994) Transfer of human chromosome 3 to an ovarian carcinoma cell line identifies three regions on 3p involved in ovarian cancer. Oncogene 9:3467–3474
Russell SE, McCluggage WG (2004) A multistep model for ovarian tumorigenesis: the value of mutation analysis in the KRAS and BRAF genes. J Pathol 203:617–619
Silva EG, Kurman RJ, Russell P, Scully RE (1996) Symposium: ovarian tumors of borderline malignancy. Int J Gynecol Pathol 15:281–302
Song MS, Song SJ, Ayad NG, Chang JS, Lee JH, Hong HK, Lee H, Choi N, Kim J, Kim H, Kim JW, Choi EJ, Kirschner MW, Lim DS (2004) The tumour suppressor RASSF1A regulates mitosis by inhibiting the APC-Cdc20 complex. Nat Cell Biol 6:129–137
Wong IH, Chan J, Wong J, Tam PK (2004) Ubiquitous aberrant RASSF1A promoter methylation in childhood neoplasia. Clin Cancer Res 10:994–1002
Xing M Cohen Y, Mambo E, Tallini G, Udelsman R, Ladenson PW, Sidransky D (2004) Early occurrence of RASSF1A hypermethylation and its mutual exclusion with BRAF mutation in thyroid tumorigenesis. Cancer Res 64:1664–1668
Yoon JH, Dammann R, Pfeifer GP (2001) Hypermethylation of the CpG island of the RASSF1A gene in ovarian and renal cell carcinomas. Int J Cancer 94:212–217
Acknowledgements
This work was supported in part by Samsung Biomedical Research Institute grant no. SBRI CA32161.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, YL., Kang, S.Y., Choi, J.S. et al. Aberrant hypermethylation of RASSF1A promoter in ovarian borderline tumors and carcinomas. Virchows Arch 448, 331–336 (2006). https://doi.org/10.1007/s00428-005-0091-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-005-0091-3