Abstract
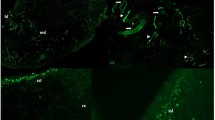
Fascin, a 55-kDa actin-bundling protein, and α-smooth muscle actin (ASMA) were immunohistochemically examined in murine normal and stimulated lymph nodes. In specific pathogen-free young female mice, a few fascin-positive cells (FPCs) were located in the sinus and surrounding tissues, but ASMA-positive cells were undetectable. Following a subcutaneous injection of sheep red blood cells, the numbers of FPCs and their dendrites increased in the paracortex, with the accumulation of activated lymphocytes. Fibroblastic reticulum cells (FRCs), endothelial cells, histiocytic cells and lymphocytes in various stages of maturation were all fascin negative. These results indicated that fascin could be a reliable marker of paracortical dendritic cells in murine lymph nodes. However, FRCs became ASMA positive. Immunoelectron microscopy showed that the FPCs were interdigitating cells and that they closely contacted with FRCs. These two types of cells and reticular fiber formed a network in the paracortex and contacted with each other. In active paracortical response, both FPCs and FRCs are also stimulated and might play a significant role in the maturation of the lymphocytes.





Similar content being viewed by others
References
Adams JC, Clelland JD, Collett GD, Matsumura F, Yamashiro S, Zhang L (1999) Cell-matrix adhesions differentially regulate fascin phosphorylation. Mol Biol Cell 10:4177–4190
Al-Alwan MM, Rowden G, Lee TDG, West A (2001) Fascin is involved in the antigen presentation activity of mature dendritic cells. J Immunol 166:338–345
Ardavín G, Martínez del Hoyo G, Martín P, Anjuère F, Arias CF, Marín AR, Ruiz S, Parrillas V, Hernández H (2001) Origin and differentiation of dendritic cells. Trends Immunol 22:691–700
Austyn JM (1996) New insights into the mobilization and phagocytic activity of dendritic cells. J Exp Med 183:1287
Branchereau J, Steinmann RM (1998) Dendritic cells and the control of immunity. Nature 392:245–252
Buelens C, Verhasselt V, De Groote D, Thielemans K, Goldman M, Willems F (1997) Human dendritic cell responses to lipopolysaccharide and CD40 ligation are differentially regulated by interleukin-10. Eur J Immunol 27:1848
Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait Yahia S (1998) Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med 188:373–386
Enzan H, Hiroi M, Saibara T, Onishi S, Yamamoto Y, Yamamoto H, Hara H (1991) Immunoelectron microscopic identification of asialo GM1-positive cells in adult rat liver. Virchows Arch 60:389–398
Grouard G, Durand I, Filgueira L, Banchereau J, Liu YJ (1996) Dendritic cells capable of stimulating T cells in germinal centers. Nature 384:364–367
Hart DN (1997) Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 90:3245
Inaba K, Pack M, Inaba M, Sakuta H, Isdell F, Steinman RM (1997) High levels of a major histocompatibility complex II-self peptide complex on dendritic cells from the T cell areas of lymph nodes. J Exp Med 186:665–672
Kapasi Z, Qin D, Kerr W, Kosco-Vilbois M, Shultz L, Tew J, Szakal A (1998) Follicullar dendritic cell (FDC) precursors in primary lymphoid tissues. J Immunol 160:1078–1084
Larsen CP, Ritchie SC, Pearson TC, Linsley PS, Lowry RP (1992) Functional expression of the costimulatory molecule, B7/BB1, on murine dendritic cell populations. J Exp Med 176:1215–1220
Mosialos G, Yamashiro S, Baughman RW, Matsudaira P, Vara L, Matsumura F, Kieff E, Birkenbach M (1994) Epstein-Barr virus infection induces expression in B lymphocytes of a novel gene encoding an evolutionarily conserved 55-kilodalton actin-bundling protein. J Virol 68:7320–7328
Pinkus GS, Pinkus JL, Langhoff E, Matsumura F, Yamashiro S, Mosialos G, Said JW (1997) Fascin, a sensitive new marker for Reed Sternberg cells of Hodgkin’s disease. Evidence for a dendritic or B cell derivation? Am J Pathol 150:543–562
Roake JA, Rao AS, Morris PJ, Larsen CP, Hankins DF, Austyn JM (1995) Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J Exp Med 191:2237
Ross R, Ross XL, Schwing J, Langin T, Reske KA (1998) The actin-bundling protein fascin is involved in the formation of dendritic processes in maturing epidermal Langerhans cells. J Immunol 160:3776–3782
Said JW, Pinkus JL, Yamashita J, Mishalani S, Matsumura F, Yamashiro S, Pinkus GS (1997) The role of follicular and interdigitating dendritic cells in HIV-related lymphoid hyperplasia: localization of fascin. Mod Pathol 10:421–427
Said JW, Pinkus JL, Shintaku IP, deVos S, Matsumura F, Yamashiro S, Pinkus GS (1998) Alterations in fascin-expressing germinal center dendritic cells in neoplastic follicles of B-cell lymphomas. Mod Pathol 11:1–5
Schürch W, Seemayer T, Gabbiani, G (1997) Myofibroblasts. In: Sternberg, S (ed) Histology for pathologists. Ravens Press, New York, pp 129–165
Steinmann RM (1991) The dendritic cell system and its role in immunogenicity. Annu Rev Immunol 9:271
Stossel TP (1993) On the crawling of animal cells. Science 260:1086–1094
Termeer CC, Hennies J, Voith U, Ahrens T, Weiss JM, Prehm P, Simon JC (2000) Oligosaccharides of hyaluronan are potent activators of dendritic cells. J Immunol 165:1863–1870
Teunissen MB, Wormmeester J, Krieg SR, Peters PJ, Vogels IM, Kapsenberg ML, Bos JD (1990) Human epidermal Langerhans cells undergo profound morphologic and phenotypical changes during in vitro culture. J Invest Dermatol 94:476
Toccanier-Pelte M, Skall O, Kapanci Y, Gabbiani G (1987) Characterization of stromal cells with myoid features in lymph nodes and spleen in normal and pathologic condition. Am J Pathol 129:109–118
Yamashiro S, Yamakita Y, Ono S, Matsumura F (1998) Fascin, an actin-bundling protein, induces membrane protrusions and increases cell motility of epithelial cells. Mol Biol Cell 9:993–1006
Yamashiro-Matsushita S, Matsumura F (1985) Purification and characterization of an F-actin-bundling protein 55-kilodalton protein from HeLa cells. J Biol Chem 260:5087–5097
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hiroi, M., Kuroda, N., Toi, M. et al. Fascin-positive dendritic cells and fibroblastic reticulum cells build a framework of T-cell areas in lymph nodes. Virchows Arch 444, 158–163 (2004). https://doi.org/10.1007/s00428-003-0939-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-003-0939-3