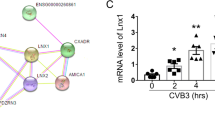
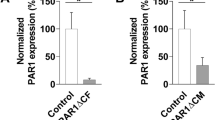
Abstract
Coxsackievirus B is the most common cause of viral myocarditis and is particularly virulent in neonates and children. Adenovirus is also a leading cause of the disease. The determinant of tropism for both viruses is considered to be the expression of coxsackievirus and adenovirus receptor (CAR) in target organs. However, developmental change and physiological localization of CAR in the heart are unknown. We examined expression levels of CAR in rat hearts by quantitative real-time polymerase chain reaction and Western blot analysis and found that CAR decreased gradually during postnatal development, although CAR was detectable, even in adults. Immunohistochemistry revealed CAR on the whole surface of cardiomyocytes in immature rat hearts. In contrast, CAR was detected predominantly on intercalated disks in the adult heart and was accumulated especially at the contact point between the cultured cardiomyocytes, even though they were prepared from the neonatal rat heart. In conclusion, CAR was expressed abundantly on the whole surface of cardiomyocytes in immature rat hearts. Both the expression level and the localization of CAR are possible determinants of the susceptibility to viral myocarditis of neonates and children.







Similar content being viewed by others
References
Angst BD, Khan LU, Severs NJ, Whitely K, Rothery S, Thompson RP, Magee AI, Gourdie RG (1997) Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ Res 80:88–94
Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW (1997) Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320–1323
Bowles NE, Towbin JA (2003) Childhood myocarditis and dilated cardiomyopathy. In: Cooper LT (ed) Myocarditis: from bench to bedside. Humana Press, Totowa, pp 559–587
Carson SD (2000) Limited proteolysis of the coxsackievirus and adenovirus receptor (CAR) on HeLa cells exposed to trypsin. FEBS Lett 484:149–152
Carson SD, Chapman NN, Tracy SM (1997) Purification of the putative coxsackievirus B receptor from HeLa cells. Biochem Biophys Res Commun 233:325–328
Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM (2001) The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci U S A 98:15191–15196
Dec GW, Fuster V (1994) Idiopathic dilated cardiomyopathy. N Engl J Med 331:1564–1575
Douglas JT, Kim M, Sumerel LA, Carey DE, Curiel DT (2001) Efficient oncolysis by a replicating adenovirus (ad) in vivo is critically dependent on tumor expression of primary ad receptors. Cancer Res 61:813–917
Fechner H, Haack A, Wang H, Wang X, Eizema K, Pauschinger M, Schoemaker R, Veghel R, Houtsmuller A, Schultheiss HP, Lamers J, Poller W (1999) Expression of coxsackie adenovirus receptor and alpha-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther 6:1520–1535
Fechner H, Noutsias M, Tschoepe C, Hinze K, Wang X, Escher F, Pauschinger M, Dekkers D, Vetter R, Paul M, Lamers J, Schultheiss HP, Poller W (2003) Induction of coxsackievirus-adenovirus-receptor expression during myocardial tissue formation and remodeling: identification of a cell-to-cell contact-dependent regulatory mechanism. Circulation 107:876–882
Hanawa H, Abe S, Hayashi M, Yoshida T, Yoshida K, Shiono T, Fuse K, Ito M, Tachikawa H, Kashimura T, Okura Y, Kato K, Kodama M, Maruyama S, Yamamoto T, Aizawa Y (2002) Time course of gene expression in rat experimental autoimmune myocarditis. Clin Sci 103:623–632
Hirschman SZ, Hammer GS. Coxsackie virus myopericarditis. A microbiological and clinical review. Am J Cardiol 34:224–232
Honda T, Saitoh H, Masuko M, Katagiri-Abe T, Tominaga K, Kozakai I, Kobayashi K, Kumanishi T, Watanabe YG, Odani S, Kuwano R (2000) The coxsackievirus-adenovirus receptor protein as a cell adhesion molecule in the developing mouse brain. Brain Res Mol Brain Res 77:19–28
Ito M, Kodama M, Masuko M, Yamaura M, Fuse K, Uesugi Y, Hirono S, Okura Y, Kato K, Hotta Y, Honda T, Kuwano R, Aizawa Y (2000) Expression of coxsackievirus and adenovirus receptor in hearts of rats with experimental autoimmune myocarditis. Circ Res 86:275–280
Leon RP, Hedlund T, Meech SJ, Li S, Schaack J, Hunger SP, Duke RC, DeGregori J (1998) Adenoviral-mediated gene transfer in lymphocytes. Proc Natl Acad Sci U S A 95:13159–13164
Martin AB, Webber S, Fricker FJ, Jaffe R, Demmler G, Kearney D, Zhang YH, Bodurtha J, Gelb B, Ni J, Bricker TB, Towbin JA (1994) Acute myocarditis. Rapid diagnosis by PCR in children. Circulation 90:330–339
Montague TJ, Lopaschuk GD, Davies NJ (1990) Viral heart disease. Chest 98:190–199
Nakagawa M, Sato A, Okagawa H, Kondo M, Okuno M, Takamatsu T (1999) Detection and evaluation of asymptomatic myocarditis in schoolchildren: report of four cases. Chest 116:340–345
Nalbantoglu J, Pari G, Karpati G, Holland PC (1999) Expression of the primary coxsackie and adenovirus receptor is downregulated during skeletal muscle maturation and limits the efficacy of adenovirus-mediated gene delivery to muscle cells. Hum Gene Ther 10:1009–1019
Nalbantoglu J, Larochelle N, Wolf E, Karpati G, Lochmuller H, Holland PC (2001) Muscle-specific overexpression of the adenovirus primary receptor CAR overcomes low efficiency of gene transfer to mature skeletal muscle. J Virol 75:4276-4282
Nicholson F, Ajetunmobi JF, Li M, Shackleton EA, Starkey WG, Illavia SJ, Muir P, Banatvala JE (1995) Molecular detection and serotypic analysis of enterovirus RNA in archival specimens from patients with acute myocarditis. Br Heart J 74:522–527
Noutsias M, Fechner H, de Jonge H, Wang X, Dekkers D, Houtsmuller AB, Pauschinger M, Bergelson J, Warraich R, Yacoub M, Hetzer R, Lamers J, Schultheiss HP, Poller W (2001) Human coxsackie-adenovirus receptor is colocalized with integrins alpha(v)beta(3) and alpha(v)beta(5) on the cardiomyocyte sarcolemma and upregulated in dilated cardiomyopathy: implications for cardiotropic viral infections. Circulation 104:275–280
Oyamada M, Kimura H, Oyamada Y, Miyamoto A, Ohshika H, Mori M (1994) The expression, phosphorylation, and localization of connexin 43 and gap-junctional intercellular communication during the establishment of a synchronized contraction of cultured neonatal rat cardiac myocytes. Exp Cell Res 212:351–358
Peters NS, Severs NJ, Rothery SM, Lincoln C, Yacoub MH, Green CR (1994) Spatiotemporal relation between gap junctions and fascia adherens junctions during postnatal development of human ventricular myocardium. Circulation 90:713–725
Pickles RJ, Fahrner JA, Petrella JM, Boucher RC, Bergelson JM (2000) Retargeting the coxsackievirus and adenovirus receptor to the apical surface of polarized epithelial cells reveals the glycocalyx as a barrier to adenovirus-mediated gene transfer. J Virol 74:6050–6057
Pisani B, Taylor DO, Mason JW (1997) Inflammatory myocardial diseases and cardiomyopathies. Am J Med 102:459–469
Poller W, Fechner H, Noutsias M, Tschoepe C, Schultheiss HP (2002) Highly variable expression of virus receptors in the human cardiovascular system Implications for cardiotropic viral infections and gene therapy. Z Kardiol 91:978–991
Reinecke H, Zhang M, Bartosek T, Murry CE (1999) Survival, integration, and differentiation of cardiomyocyte grafts: a study in normal and injured rat hearts. Circulation 100:193–202
Rosenberg HS, McNamara DG (1964) Acute myocarditis in infancy and childhood. Prog Cardiovasc Dis 7:179–197
Simpson P, Savion S (1982) Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circ Res 50:101–116
Thoelen I, Magnusson C, Tagerud S, Polacek C, Lindberg M, Van Ranst M (2001) Identification of alternative splice products encoded by the human coxsackie-adenovirus receptor gene. Biochem Biophys Res Commun 287:216–222
Tomko RP, Xu R, Philipson L (1997) HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci U S A 94:3352–3356
Walters RW, Grunst T, Bergelson JM, Finberg RW, Welsh MJ, Zabner J (1999) Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem 274:10219–10226
Walters RW, van’t Hof W, Yi SM, Schroth MK, Zabner J, Crystal RG, Welsh MJ (2001) Apical localization of the coxsackie-adenovirus receptor by glycosyl-phosphatidylinositol modification is sufficient for adenovirus-mediated gene transfer through the apical surface of human airway epithelia. J Virol 75:7703–7711
Walters RW, Freimuth P, Moninger TO, Ganske I, Zabner J, Welsh MJ (2002) Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell 110:789–799
Wan YY, Leon RP, Marks R, Cham CM, Schaack J, Gajewski TF, DeGregori J (2000) Transgenic expression of the coxsackie/adenovirus receptor enables adenoviral-mediated gene delivery in naive T cells. Proc Natl Acad Sci U S A 97:13784–13789
Young JAT (2001) Virus entry and uncoating. In: Kimpe DM, Howley PM (eds) Fieles virology. Lippincott Williams & Wilkins, Philadelphia, pp 87–103
Acknowledgement
This study was supported in part by a grant for research on specific diseases from the Ministry of Health, Labor and Welfare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kashimura, T., Kodama, M., Hotta, Y. et al. Spatiotemporal changes of coxsackievirus and adenovirus receptor in rat hearts during postnatal development and in cultured cardiomyocytes of neonatal rat. Virchows Arch 444, 283–292 (2004). https://doi.org/10.1007/s00428-003-0925-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-003-0925-9