Abstract
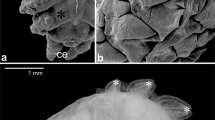
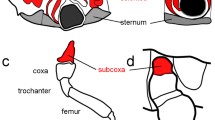
The abdominal appendages on male Themira biloba (Diptera: Sepsidae) are complex novel structures used during mating. These abdominal appendages superficially resemble the serially homologous insect appendages in that they have a joint and a short segment that can be rotated. Non-genital appendages do not occur in adult pterygote insects, so these abdominal appendages are novel structures with no obvious ancestry. We investigated whether the genes that pattern the serially homologous insect appendages have been co-opted to pattern these novel abdominal appendages. Immunohistochemistry was used to determine the expression patterns of the genes extradenticle (exd), Distal-less (Dll), engrailed (en), Notch, and the Bithorax Complex in the appendages of T. biloba during pupation. The expression patterns of Exd, En, and Notch were consistent with the hypothesis that a portion of the patterning pathway that establishes the coxopodite has been co-opted to pattern the developing abdominal appendages. However, Dll was only expressed in the bristles of the developing appendages and not the proximal–distal axis of the appendage itself. The lack of Dll expression indicates the absence of a distal domain of the appendage suggesting that sepsid abdominal appendages only use genes that normally pattern the base of segmental appendages.






Similar content being viewed by others
References
Abouheif E (1997) Developmental genetics and homology: a hierarchical approach. TREE 12:405–408
Abzhanov A, Kaufman TC (2000) Homologs of Drosophila appendage genes in the patterning of arthropod limbs. Dev Biol 227:673–689
Abu-Shaar M, Mann RS (1998) Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development 125:3821–3830
Angelini DR, Kauffman TC (2005) Insect appendages and comparative ontogenetics. Dev Biol 286:57–77
Artavanis-Tsakonas S, Matsuno K, Fortini ME (1995) Notch signaling. Science 268:225–232
Aspland SE, White RAH (1997) Nucleocytoplasmic localization of extradenticle protein is spatially regulated throughout development in Drosophila. Development 124:741–747
Bainbridge SP, Bownes M (1981) Staging the metamorphosis of Drosophila melanogaster. J Embryol Exp Morphol 66:57–80
Bishop SA, Klein T, Arias AM, Couso JP (1999) Composite signalling from Serrate and Delta establishes leg segments in Drosophila through Notch. Development 126:2993–3003
Bowsher JH, Nijhout HF (2007) Evolution of novel abdominal appendages in a sepsid fly from histoblasts, not imaginal discs. Evol Dev 9(4):347–354
Boxshall GA (2004) The evolution of arthropod limbs. Biol Rev 79:253–300
Campbell G, Tomlinson A (1998) The roles of the homeobox genes aristaless and Distal-less in patterning the legs and wings of Drosophila. Development 125:4483–4493
de Celis JF, Tyler DM, de Celis J, Bray SJ (1998) Notch signaling mediates segmentation of the Drosophila leg. Development 125:4617–4626
Dickinson WJ (1995) Molecules and morphology: where’s the homology. Trends Genet 11:119–121
Eberhard WG (2001) Multiple origins of a major novelty: moveable abdominal lobes in male sepsid flies (Diptera: Sepsidae), and the question of developmental constraints. Evol Dev 3:206–222
Eberhard WG (2003) Sexual behavior and morphology of Themira minor (Diptera: Sepsidae) males and the evolution of male sternal lobes and genitalic surstyli. Can Entomol 135:569–581
Fehon RG, Kooh PJ, Rebay I, Regan CL, Xu T, Muskavitch MAT, Artavanis-Tsakonas S (1990) Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell 61:523–534
Ganfornia MD, Sanchez D (1999) Generation of evolutionary novelty by functional shift. BioEssays 21:432–439
Gonzalez-Crespo S, Morata G (1995) Control of Drosophila adult pattern by extradenticle. Development 121:2117–2125
Hartenstein V, Posakony JW (1989) Development of adult sensilla on the wing and notum of Drosophila melanogaster. Development 107:389–405
Hennig W (1949) Sepsidae. In Lindner E (ed) Die Fliegen der Palaearktischen Region. E Schweizerbart’sche Verlagsbuchhandlung, Stuttgart, Germany, pp 1–91
Kelsh R, Weinzierl ROJ, White RAH, Akam M (1994) Homeotic gene expression in the locust Schistocerca: an antibody that detects conserved epitopes in ultrabithorax and abdominal-A proteins. Dev Gen 15:19–31
Kopp A, Muskavitch MAT, Duncan I (1997) The roles of hedgehog and engrailed in patterning adult abdominal segments of Drosophila. Development 124:3703–3714
Kopp A, Duncan I (2002) Anterioposterior patterning in adult abdominal segments of Drosophila. Dev Biol 242:15–30
Lachmann A (1991) Vergleichende Untersuchung zum Lebenszyklus der kuhdungbewohnenden Sphaeroceridenarten Chaetopodella scutellaris (Haliday, 1836) und Coproica lugubris (Haliday, 1836). Deutsche Entomologische Zeitschrift 38:197–210
Lai EC (2004) Notch signaling: control of cell communication and cell fate. Development 131(5):965–973
Lawrence PA, Struhl G (1996) Morphogens, compartments, and pattern: lessons from Drosophila? Cell 85:951–961
Lewis DL, DeCamillis M, Bennett RL (2000) Distinct roles of the homeotic genes Ubx and abd-A in beetle embryonic abdominal appendage development. Proc Natl Acad Sci USA 97(9):4504–4509
Lowe CJ, Wray GA (1997) Radical alterations in the roles of homeobox genes during echinoderm evolution. Nature 389:718–721
Mardon G, Solomon NM, Rubin GM (1994) dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development 120:3473–3486
Mayr E (1960) The emergence of evolutionary novelties. In: Tax S (ed) Evolution after Darwin. University of Chicago Press, Chicago, pp 349–380
Meier R (1995) Cladistic analysis of the Sepsidae (Cyclorrhapha: Diptera) based on a comparative scanning electron microscopic study of larvae. Syst Entomol 20:99–128
Meier R (1996) Larval morphology of the Sepsidae (Diptera: Sciomyziodea), with a cladistic analysis using adult and larval characters. Bulletin of the AMNH 228:1–147
Mirth C, Akam M (2002) Joint development in the Drosophila leg: cell movements and cell populations. Dev Biol 246:391–406
Mittman B, Scholtz G (2001) Distal-less expression in embryos of Limulus polyphemus (Chelicerata, Xiphosura) and Lepisma saccharina (Insecta, Zygentoma) suggests a role in the development of mechanoreceptors, chemoreceptors, and the CNS. Dev Genes Evol 211:232–243
Moczek AP, Rose D, Sewell W, Kesselring BR (2006) Conservation, innovation, and the evolution of horned beetle diversity. Dev Genes Evol 216:655–665
Müller GB, Wagner GP (1991) Novelty in evolution: restructuring the concept. Annu Rev Ecol Syst 22:229–256
Nagy LM, Williams TA (2001) Comparative limb development as a tool for understanding the evolutionary diversification of limbs in arthropods: challenging the modularity paradigm. In: Wagner GP (ed) The character concept in evolutionary biology. Academic, San Diego, pp 455–488
Palopoli MF, Patel NH (1998) Evolution of the interaction between Hox genes and a downstream target. Curr Biol 8:587–590
Panganiban G, Nagy L, Carroll SB (1994) The role of the Distal-less gene in the development and evolution of insect limbs. Curr Biol 4:671–675
Panganiban G, Irvine SM, Lowe C, Roehl H, Corley LS, Sherbon B, Grenier JK, Fallon JF, Kimble J, Walker M, Wray GA, Swalla BJ, Martindale MQ, Carroll SB (1997) The origin and evolution of animal appendages. Proc Natl Acad Sci USA 94:5162–5166
Patel NM, Martin-Blanco E, Coleman KG, Poole SJ, Ellis MC, Kornberg TB, Goodman CS (1989) Expression of engrailed proteins in arthropods, annelids, and chordates. Cell 58:955–968
Pont AC (1979) Sepsidae: Diptera, Cyclorrhapha, Acalyptrata. In: Fitton MG (ed) Handbooks for the identification of British insects. Royal Entomological Society of London, London, pp 1–35
Popadic A, Panganiban G, Rusch D, Shear WA, Kaufman TC (1998) Molecular evidence for the gnathobasic derivation of arthropod mandibles and for the appendicular origin of the labrum and other structures. Dev Genes Evol 208:142–150
Posakony JW (1994) Nature versus nurture: asymmetric cell divisions in Drosophila bristle development. Cell 76:415–418
Prpic NM, Wigand B, Damen WG, Klingler M (2001) Expression of dachshund in wild-type and Distal-less mutant Tribolium corroborates serial homologies in insect appendages. Dev Genes Evol 211:467–477
Rauskolb C (2001) The establishment of segmentation in the Drosophila leg. Development 128:4511–4521
Rauskolb C, Smith KM, Peifer M, Wieschaus E (1995) extradenticle determines segmental identities throughout Drosophila development. Development 121:3663–3673
Roth VL (1988) The biological basis of homology. In: Humphries CJ (ed) Ontogeny and systematics. Columbia University Press, New York, pp 1–26
Scholtz G, Mittmann B, Gerberding M (1998) The pattern of Distal-less expression in the mouthparts of crustaceans, myriapods and insects: new evidence for the gnathobasic mandible and the common origin of Mandibulata. Int J Dev Biol 42:801–810
Schoppmeier M, Damen WGM (2001) Double-stranded RNA interference in the spider Cupiennius salei: the role of Distal-less is evolutionarily conserved in arthropod appendage formation. Dev Genes Evol 211:76–82
Snodgrass RE (1935) Principles of insect morphology. McGraw-Hill Book Company, New York
Suzuki Y, Palopoli MF (2001) Evolution of insect abdominal appendages: are prolegs homologous or convergent traits? Dev Genes Evol 211:486–492
Svácha P (1992) What are and what are not imaginal discs: reevaluation of some basic concepts (Insecta, Holometabola). Dev Biol 154:101–117
Tanaka K, Truman JW (2005) Development of the adult leg epidermis in Manduca sexta: contribution of different larval cell populations. Dev Genes Evol 215(2):78–89
True JR, Carroll SB (2002) Gene co-option in physiological and morphological evolution. Annu Rev Cell Dev Biol 18:53–80
Vachon G, Cohen B, Pfeifle C, McGuffin ME, Bota J, Cohen SM (1992) Homeotic genes of the Bithorax Complex repress limb development in the abdomen of the Drosophila embryo through the target gene Distal-less. Cell 71:437–450
Warren RW, Nagy L, Selegue J, Gates J, Carroll S (1994) Evolution of homeotic gene regulation and function in flies and butterflies. Nature 372:458–461
Williams TA, Nulsen C, Nagy LM (2002) A complex role for Distal-less in crustacean appendage development. Dev Biol 241:302–312
Zipursky SL, Venkatesh TR, Teplow DB, Benzer S (1984) Neural development in the Drosophila retina: monoclonal antibodies as molecular probes. Cell 36:15–26
Acknowledgments
We would like to thank Rudolf Meier for sharing his culture of T. biloba, and Bill Eberhard for sharing his knowledge of sepsid behavior and rearing. Thank you to Rob White, Nipam Patel, and Sean Carroll for providing antibodies. We would like to thank Lisa Nagy, Yui Suzuki, Robin Smith, and two anonymous reviewers for their comments on the manuscript. Laura Grunert provided critical technical assistance. We would also like to thank Maple View Farm in Chapel Hill, NC and the University of Arizona Agricultural Center for providing cow dung. This work was funded by the Department of Biology at Duke University (JHB and HFN), National Science Foundation grant IBN-0315897 (HFN), and the Center for Insect Science at the University of Arizona through the National Institute of Health Training Grant 1K12 GM000708 (JHB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Simpson
Rights and permissions
About this article
Cite this article
Bowsher, J.H., Nijhout, H.F. Partial co-option of the appendage patterning pathway in the development of abdominal appendages in the sepsid fly Themira biloba . Dev Genes Evol 219, 577–587 (2009). https://doi.org/10.1007/s00427-010-0319-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-010-0319-3