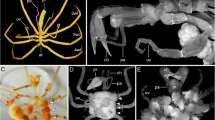
Abstract
To examine the evolution of development and put it into a phylogenetic context, it is important to have, in addition to a model organism like Drosophila, more insights into the huge diversity of arthropod morphologies. In recent years, the malacostracan crustacean Porcellio scaber Latreille, 1804 has become a popular animal for studies in evolutionary and developmental biology, but a detailed and complete description of its embryonic development is still lacking. Therefore, the embryonic development of the woodlouse P. scaber is described in a series of discrete stages easily identified by examination of living animals and the widely used technique of nuclei staining on fixed specimens. It starts with the first cleavage of the zygote and ends with a hatched manca that eventually leaves the mother’s brood pouch. Classical methods like normal light microscopy, scanning electron microscopy and fluorescence microscopy are used, in addition to confocal LCM and computer-aided 3D reconstruction in order to visualise important processes during ontogeny. The purpose of these studies is to offer an easy way to define the different degrees of development for future comparative analyses of embryonic development amongst crustaceans in particular, as well as between different arthropod groups. In addition, several aspects of Porcellio embryonic development, such as the mouth formation, limb differentiations and modifications or the formation of the digestive tract, make this species particularly interesting for future studies in evolutionary and developmental biology.









Similar content being viewed by others
References
Abzhanov A, Kaufman TC (1999a) Homeotic genes and the arthropod head: expression patterns of the labial, proboscipedia, and Deformed genes in crustaceans and insects. PNAS USA 96:10224–10229
Abzhanov A, Kaufman TC (1999b) Novel regulation of the homeotic gene Scr associated with a crustacean leg-to-maxilliped appendage transformation. Development 126:1121–1128
Abzhanov A, Kaufman TC (2000a) Crustacean (malacostracan) Hox genes and the evolution of the arthropod trunk. Development 127:2239–2249
Abzhanov A, Kaufman TC (2000b) Evolution of distinct expression patterns for engrailed paralogues in higher crustaceans (Malacostraca). Dev Genes Evol 210:493–506
Abzhanov A, Kaufman TC (2000c) Homologs of Drosophila appendage genes in the patterning of arthropod limbs. Dev Biol 227:673–689
Abzhanov A, Kaufman TC (2004) Hox genes and tagmatization of the higher Crustacea (Malacostraca). In: Scholtz G (ed) Evolutionary developmental biology of Crustacea. A.A. Balkema, Lisse, pp 43–71
Alwes F (2008) Cell lineage studies in Crustacea—Aspects of the early development and germ layer formation in Meganyctiphanes norvegica (Malacostraca, Euphausiacea) and Bythotrephes longimanus (Cladocera, Branchiopoda). Humboldt-University Berlin. Ph.D. thesis, pp 109
Anderson DT (1973) Embryology and phylogeny in annelids and arthropods. Pergamon, Oxford, p 495
Araujo PB, Quadros AF, Augusto MM, Bond-Buckup G (2004) Postmarsupial development of Atlantoscia floridana (van Name, 1940) (Crustacea, Isopoda, Oniscidea): sexual differentiation and size at onset of sexual maturity. Inv Repr Dev 45:221–230
Averof M, Cohen SM (1997) Evolutionary origin of insect wings from ancestral gills. Nature 385:627–630
Bitsch J (2001) The hexapod appendage: basic structure, development and origin. Ann Soc Entomol Fr (NS) 37:175–193
Borradaile LA (1926) Notes upon crustacean limbs. Ann Mag Nat Hist Ser 9 17(98):193–213
Boxshall GA (2004) The evolution of arthropod limbs. Biol Rev 79:253–300
Boxshall GA, Jaume D (2009) Exopodites, epipodites and gills in crustaceans. Arth Syst Phyl 67:229–254
Brena C, Liu PZ, Minelli A, Kaufman TC (2005) Abd-B expression in Porcellio scaber Latreille, 1804 (Isopoda: Crustacea): conserved pattern versus novel roles in development and evolution. Evol Dev 7:42–50
Browne WE, Price AL, Gerberding M, Patel NH (2005) Stages of embryonic development in the amphipod crustacean, Parhyale hawaiensis. Genesis 42:124–149
Brum PED, Araujo PB (2007) The manca stages of Porcellio dilatatus Brandt (Crustacea, Isopoda, Oniscidea). Rev Brasil Zool 24:493–502
Bullar JF (1878) On the development of the parasitic Isopoda. Phil Trans Roy Soc London 169:505–521
Calman WT (1909) Crustacea. In: Lankester ER (ed) A treatise on zoology. Adam and Charles Black, London, p 346
Campos-Ortega JA, Hartenstein V (1997) The embryonic development of Drosophila melanogaster. Springer, Berlin, p 405
Damen WGM, Saridaki T, Averof M (2002) Diverse adaptations of an ancestral gill: a common evolutionary origin for wings, breathing organs, and spinnerets. Cur Biol 12:1711–1716
de Celis JF, Llimargas M, Casanova J (1995) Ventral veinless, the gene encoding the Cf1 a transcription factor, links positional information and cell differentiation during embryonic and imaginal development in Drosophila melanogaster. Development 121:3405–3416
Dohle W, Scholtz G (1988) Clonal analysis of the crustacean segment: the discordance between genealogical and segmental borders. Development Suppl 104:147–160
Dohle W, Scholtz G (1997) How far does cell lineage influence cell fate specification in crustacean embryos? Sem Cell Dev Biol 8:379–390
Dohle W, Gerberding M, Hejnol A, Scholtz G (2004) Cell lineage, segment differentiation, and gene expression in crustaceans. In: Scholtz G (ed) Evolutionary developmental biology of Crustacea. Crustacean issues 15. A.A. Balkema, Lisse, pp 95–133
Dohrn A (1866) Die embryonale Entwicklung des Asellus aquaticus. Z Wiss Zool 17:221–278
Drobne D (1997) Terrestrial isopods—a good choice for toxicity testing of pollutants in the terrestrial environment. Environ Toxicol Chem 16:1159–1164
Franch-Marro X, Martin N, Averof M, Casanova J (2006) Association of tracheal placodes with leg primordia in Drosophila and implications for the origin of insect tracheal systems. Development 133:785–790
Gerberding M (1994) Superfizielle Furchung, Bildung des Keimstreifs und Differenzierung von Neuroblasten bei Leptodora kindti Focke 1844 (Cladocera, Crustacea). Humboldt-University Berlin, Diploma thesis, pp 60
Goodrich AL (1939) The origin and fate of the entoderm elements in the embryogeny of Porcellio laevis Latr. and Armadillidium nasatum B.L. (Isopoda). J Morph 64:401–429
Gruner HE (1954) Über das Coxalglied der Peripoden der Isopoden. Zool Anz 152:312–317
Gruner H-E (1965) Krebstiere oder Crustacea, V. Isopoda (erster Teil). In: Dahl F (ed) Die Tierwelt Deutschlands, 51. Teil. Gustav Fischer, Jena, p 149
Gruner H-E (1966) Krebstiere oder Crustacea, V. Isopoda (zweiter Teil). In: Dahl F (ed) Die Tierwelt Deutschlands, 53. Teil. Gustav Fischer, Jena, p 230
Gruner H-E (1993) Klasse Crustacea. In: Gruner H-E (ed) Lehrbuch der speziellen Zoologie. Gustav Fischer, Jena, pp 448–1009, Band 1: Wirbellose Tiere, 4. Teil: Arthropoda (ohne Insecta)
Hahnenkamp L (1974) Die Bildung und Differenzierung des Keimstreifs der Asseln (Isopoda) und anderer höherer Krebse. Eine vergleichend-embryologische Studie. Berlin, Freie Universität. Hausarbeit für die erste (wissenschaftliche) Staatsprüfung, pp 180
Hames CAC, Hopkin SP (1989) The structure and function of the digestive system of terrestrial isopods. J Zool 217:599–627
Hansen HJ (1925) Studies on Arthropoda II. On the comparative morphology of the appendages in the Arthropoda. A. Crustacea. Gyldendalske Boghandel, Copenhagen, p 157
Hartenstein V (1993) Atlas of Drosophila development. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, p 57
Havemann J, Müller U, Berger J, Schwarz H, Gerberding M, Moussian B (2008) Cuticle differentiation in the embryo of the amphipod crustacean Parhyale hawaiensis. Cell Tissue Res 332:359–370
Hejnol A, Scholtz G (2004) Clonal analysis of Distal-less and engrailed expression patterns during early morphogenesis of uniramous and biramous crustacean limbs. Dev Genes Evol 214:473–485
Hejnol A, Schnabel R, Scholtz G (2006) A 4D-microscopic analysis of the germ band in the isopod crustacean Porcellio scaber (Peracarida, Malacostraca)—developmental and phylogenetic implications. Dev Genes Evol 216:755–767
Hickman VV (1937) The embryology of the syncarid crustacean, Anaspides tasmaniae. Paps Proc Roy Soc Tasmania 1–35
Hoese B (1981) Morphologie und Funktion des Wasserleitungssystems der terrestrischen Isopoden (Crustacea, Isopoda, Oniscoidea). Zoomorphology 98:135–167
Hoese B (1983) Structures and development of the lungs in Tylidae (Crustacea, Isopoda, Oniscidoidea). Zool Jb Anat 109:487–501
Hoese B, Janssen HH (1989) Morphological and physiological studies on the marsupium in terrestrial isopods. Ital J Zool 4:153–173
Holdich DM (1973) The midgut/hindgut controversy in isopods. Crustaceana 24:211–214
Jaume D (2001) A new atlantasellid isopod (Asellota: Aselloidea) from the flooded coastal karst of the Dominican Republic (Hispaniola): evidence for an exopod on a thoracic limb and biogeographical implications. J Zool 255:221–233
Kajishima T (1952) Experimental studies on the embryonic development of the isopod crustacean, Megaligia exotica Roux. Annat Zool Jap 25:172–181
Knopf F, Koenemann S, Schram FR, Wolff C (2006) The urosome of the pan- and Peracarida. Cont Biol 75:1–21
Kreissl S, Uber A, Harzsch S (2008) Muscle precursor cells in the developing limbs of two isopods (Crustacea, Peracarida): an immunohistochemical study using a novel monoclonal antibody against myosin heavy chain. Dev Genes Evol 218:253–265
Lauterbach KE (1975) Über die Herkunft der Malacostraca (Crustacea). Zool Anz 194:165–179
Liu Y, Maas A, Waloszek D (2009) Early development of the anterior body region of the grey widow spider Latrodectus geometricus Koch, 1841 (Theridiidae, Araneae). Arthr Struct Dev 38:401–416
Manton SM (1928) On the embryology of a mysid crustacean, Hemimysis lamornae. Phil Trans Roy Soc London 216:363–463
McMurrich JP (1895) Embryology of the isopod Crustacea. J Morph 11:63–154
Morgan TH (1891) A contribution to the embryology and phylogeny of the pycnogonids. Stud Biol Lab J Hopkins Univ 5:1–76
Nair SG (1956) On the embryology of the isopod Irona. J Dev Exp Morph 4:1–33
Nusbaum J (1891) Beiträge zur Embryologie der Isopoden. Zool Anz 11:42–49
Powell CVL, Halcrow K (1985) Formation of the epicuticle in a marine isopod, Idotea baltica (Pallas). J Crust Biol 5:439–448
Richter S, Scholtz G (2001) Phylogenetic analysis of the Malacostraca (Crustacea). J Zool Syst Evol Res 39:113–136
Samter M (1900) Studien zur Entwicklungsgeschichte der Leptodora hyalina Lillj. Z Wiss Zool 68:169–260
Schmidt C, Wägele JW (2001) Morphology and evolution of respiratory structures in the pleopod exopodites of terrestrial Isopoda (Crustacea, Isopoda, Oniscidea). Act Zool 82:315–330
Scholl G (1963) Embryologische Untersuchungen an Tanaidaceen (Heterotanais oerstedi Kröyer). Zool Jb Anat 80:500–554
Scholtz G (1995) Expression of the engrailed gene reveals nine putative segment-anlagen in the embryonic pleon of the freshwater crayfish Cherax destructor (Crustacea, Malacostraca, Decapoda). Biol Bull 188:157–165
Scholtz G (1997) Cleavage, germ band formation and head segmentation: the ground pattern of the Euarthropoda. In: Fortey RA, Thomas RH (eds) Arthropod relationships, vol 24. Chapman & Hall, London, pp 317–332
Scholtz G, Dohle W (1996) Cell lineage and cell fate in crustacean embryos—a comparative approach. Int J Dev Biol 40:211–220
Scholtz G, Wolff C (2002) Cleavage, gastrulation, and germ disc formation in the amphipod Orchestia cavimana (Crustacea, Malacostraca, Peracarida). Cont Biol 71:9–28
Schram FR (1986) Crustacea. Oxford Press, New York, p 606
Shiino SM (1942) Studies on the embryology of Squilla oratoria de Haan. Mem Coll Sci Kyoto Imp Univ Series B 17:77–174
Snodgrass RE (1952) A textbook of arthropod anatomy. Comstock, Ithaca, p 363
Strömberg J-O (1965) On the embryology of the isopod Idotea. Ark Zool 17:421–467
Strömberg J-O (1967) Segmentation and organogenesis in Limnoria lignorum (Rathke) (Isopoda). Ark Zool 20:91–139
Strömberg J-O (1971) Contribution to the embryology of bopryid isopods; with special reference to Bopyroides, Hemiarthrus and Pseudione (Isopoda, Epicaridea). Sarsia 47:1–47
Strömberg J-O (1972) Cyathura polita (Crustacea, Isopoda), some embryological notes. Bull Mar Sci 22:463–482
Strus J, Drobne D, Licar P (1995) Comparative anatomy and functional aspects of the digestive system in amphibious and terrestrial isopods (Isopoda: Oniscidea). In: Alikhan MA (ed) Crustacean Issues 9; terrestrial isopod biology. A.A. Balkema, Rotterdam, pp 15–23
Strus J, Klepal W, Repina J, Tusek-Znidaric M, Milatovic M, Pipan Z (2008) Ultrastructure of the digestive system and the fate of midgut during embryonic development in Porcellio scaber (Crustacea: Isopoda). Arthr Struct Dev 37:287–98
Takashima S, Mkrtchyan M, Younossi-Hartenstein A, Merriam JR, Hartenstein V (2008) The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature 454:651–656
Thiele J (1905) Betrachtungen über die Phylogenie der Crustaceenbeine. Z Wiss Zool 82:445–471
Tomescu N, Craciun C (1987) Postembryonic ontogenetic development in Porcellio scaber (Crustacea, Isopoda). Pedobiologia 30:345–350
Ungerer P, Wolff C (2005) External morphology of limb development in the amphipod Orchestia cavimana (Crustacea, Malacostraca, Peracarida). Zoomorphology 124:89–99
Wägele JW (1992) Isopoda. In: Harrison FW, Humes AG (eds) Microscopic anatomy of invertebrates, Crustacea, vol 9. Wiley-Liss, New York, pp 529–617
Walossek D (1999) On the Cambrian diversity of Crustacea. Crustaceans and the biodiversity crises. Proceedings of the 4th International Crustacean Congress. Brill, Amsterdam, pp 3–27
Walossek D (2003) Cambrian ‘Orsten’-type arthropods and the phylogeny of Crustacea. The new panorama of animal evolution. Proceedings of the 18th International Congress of Zoology. Pensoft, Athens, pp 71–88
Weygoldt P (1958) Die Embryonalentwicklung des Amphipoden Gammarus pulex pulex (L). Zool Jb Anat 77:51–110
Whitington PM, Leach D, Sandeman R (1993) Evolutionary change in neural development within the arthropods: axogenesis in the embryo of two crustaceans. Development 118:449–461
Wilson GDF (2009) The phylogenetic position of the Isopoda in the Peracarida (Crustacea: Malacostraca). Arthr Syst Phyl 67:159–198
Wolff C, Scholtz G (2008) The clonal composition of biramous and uniramous arthropod limbs. Proc R Soc B 275:1023–1028
Zidar P, Van Gestel CAM, Strus J (2009) Single and joint effects of Zn and Cd on Porcellio scaber (Crustacea, Isopoda) exposed to artificially contaminated food. Ecotoxicol Environ Saf 72:2075–2082
Acknowledgements
I thank R. Mbacke for the help with collecting specimens and G. Drescher (Natural History Museum, Berlin) for the support in using the SEM. I also thank Kristen Panfilio and the two reviewers for the helpful advice and Greg Edgecombe for improving the English.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Roth
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file. Mpg movie: Gut_peristaltic_stage15: The time lapse movie (normal light microscope) shows the peristaltic contractions of the midgut anlagen in a living Porcellio embryo (lateral view, anterior is left). Note that only the posterior part of the midgut is moving. While the midgut tubes elongate more and more during development, the contractions are more frequent (approximately two times per minute). (MPG 498 kb)
Rights and permissions
About this article
Cite this article
Wolff, C. The embryonic development of the malacostracan crustacean Porcellio scaber (Isopoda, Oniscidea). Dev Genes Evol 219, 545–564 (2009). https://doi.org/10.1007/s00427-010-0316-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-010-0316-6