Abstract
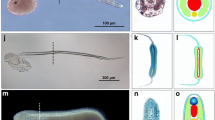
Cephalochordates, the most basal extant group in the phylum Chordata, are represented chiefly by about 20 species of the genus Branchiostoma, commonly called amphioxus or lancelets. In recent years, insights into the evolutionary origin of the vertebrates have been gained from molecular genetic studies during the development of three of these amphioxus species (Branchiostoma floridae in North America, Branchiostoma lanceolatum in Europe, and Branchiostoma belcheri in East Asia). In spite of an estimated divergence time of 100–200 Myr among these species, all three are remarkably similar morphologically, and students of amphioxus have tacitly assumed that such resemblances arise during ontogeny from nearly identical networks of developmental genes. We felt that this assumption needed to be reexamined because instances are known—even in comparisons of closely related species—where characters seeming homologous on the basis of morphology actually develop under the control of conspicuously divergent genetic programs (a phenomenon termed “genetic piracy”). In the present work, we tested the hypothesis that morphological similarities reflect strict conservation of developmentally important genes’ expression patterns in order to assess whether the developmental genetics of different amphioxus species show evidence of genetic piracy. To these ends, we cloned 18 genes implicated in different developmental functions in B. lanceolatum and compared their gene expression patterns with the known expression patterns of their orthologous genes in B. floridae. We show that, for the most part, conservation of gene expression parallels that of morphology in these two species. We also identified some differences in gene expression, likely reflecting experimental sensitivity, with the exception of Pax1/9, which may result from true developmental specificities in each amphioxus species. Our results demonstrate that morphological conservation reflects stasis in developmental gene expression patterns and find no evidence for genetic piracy. Thus, different species of amphioxus appear to be very similar, not only morphologically, but also in the genetic programs directing the development of their structural features. Moreover, we provide the first catalogue of gene expression data for the European species, B. lanceolatum.



Similar content being viewed by others
References
Boorman CJ, Shimeld SM (2002) Pitx homeobox genes in Ciona and amphioxus show left–right asymmetry is a conserved chordate character and define the ascidian adenohypophysis. Evol Dev 4(5):354–365
Cañestro C, Albalat R et al (2002) Ascidian and amphioxus Adh genes correlate functional and molecular features of the ADH family expansion during vertebrate evolution. J Mol Evol 54(1):81–89
Darras S, Nishida H (2001) The BMP/CHORDIN antagonism controls sensory pigment cell specification and differentiation in the ascidian embryo. Dev Biol 236(2):271–288
De Beer G (1958) Embryos and ancestors. Clarendon, Oxford
Dehal P, Boore JL (2005) Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol 3(10):e314
Delsuc F, Brinkmann H et al (2006) Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439(7079):965–968
Fuentes M, Schubert M et al (2004) Preliminary observations on the spawning conditions of the European amphioxus (Branchiostoma lanceolatum) in captivity. J Exp Zoolog B Mol Dev Evol 302(4):384–391
Fuentes M, Benito E et al (2007) Insights into spawning behavior and development of the european amphioxus (Branchiostoma lanceolatum). J Exp Zoolog B Mol Dev Evol 308(4):484–493
Galtier N, Gouy M et al (1996) SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci 12:543–548
García-Fernández J, Holland PWH (1994) Archetypal organization of the amphioxus Hox gene cluster. Nature 370(6490):563–566
Holland ND, Chen J (2001) Origin and early evolution of the vertebrates: new insights from advances in molecular biology, anatomy, and palaeontology. Bioessays 23(2):142–151
Holland LZ, Holland ND (1999) Chordate origins of the vertebrate central nervous system. Curr Opin Neurobiol 9(5):596–602
Holland LZ, Pace DA et al (1995a) Sequence and expression of amphioxus alkali myosin light chain (AmphiMLC-alk) throughout development: implications for vertebrate myogenesis. Dev Biol 171(2):665–676
Holland ND, Holland LZ et al (1995b) An amphioxus Pax gene, AmphiPax-1, expressed in embryonic endoderm, but not in mesoderm: implications for the evolution of class I paired box genes. Mol Mar Biol Biotechnol 4(3):206–214
Holland PW, Koschorz B et al (1995c) Conservation of Brachyury (T) genes in amphioxus and vertebrates: developmental and evolutionary implications. Development 121(12):4283–4291
Holland LZ, Holland PWH et al (1996) Revealing homologies between body parts of distantly related animals by in situ hybridization to developmental genes: amphioxus versus vertebrates. In: Ferraris JD, Palumbi SR (eds) Molecular zoology: advances, strategies, and protocols. Wiley-Liss, New York, pp 267–282. (473–483)
Holland LZ, Schubert M et al (1999) AmphiPax3/7, an amphioxus paired box gene: insights into chordate myogenesis, neurogenesis, and the possible evolutionary precursor of definitive vertebrate neural crest. Evol Dev 1(3):153–165
Holland LZ, Schubert M et al (2000) Evolutionary conservation of the presumptive neural plate markers AmphiSox1/2/3 and AmphiNeurogenin in the invertebrate chordate amphioxus. Dev Biol 226(1):18–33
Holland LZ, Rached LA et al (2001) Characterization and developmental expression of the amphioxus homolog of Notch (AmphiNotch): evolutionary conservation of multiple expression domains in amphioxus and vertebrates. Dev Biol 232(2):493–507
Kiontke K, Barriere A et al (2007) Trends, stasis, and drift in the evolution of nematode vulva development. Curr Biol 17(22):1925–1937
Knight RD, Panopoulou GD et al (2000) An amphioxus Krox gene: insights into vertebrate hindbrain evolution. Dev Genes Evol 210(10):518–521
Kozmik Z, Holland ND et al (1999) Characterization of an amphioxus paired box gene, AmphiPax2/5/8: developmental expression patterns in optic support cells, nephridium, thyroid-like structures and pharyngeal gill slits, but not in the midbrain–hindbrain boundary region. Development 126(6):1295–1304
Lowe CJ, Wray GA (1997) Radical alterations in the roles of homeobox genes during echinoderm evolution. Nature 389(6652):718–721
Marzoli A, Renne PR et al (1999) Extensive 200-million-year-Old continental flood basalts of the central Atlantic magmatic province. Science 284(5414):616–618
Miller-Bertoglio VE, Fisher S et al (1997) Differential regulation of chordin expression domains in mutant zebrafish. Dev Biol 192(2):537–550
Minelli A (1998) Molecules, developmental modules, and phenotypes: a combinatorial approach to homology. Molecular Phylogeny and Evolution 9:340–347
Nohara M, Nishida M et al (2004) Ancient phylogenetic separation between Pacific and Atlantic cephalochordates as revealed by mitochondrial genome analysis. Zoolog Sci 21(2):203–210
Pallas PS (1774) Spicilegia zoologica, quibus novae imprimus et obscurae animalium species iconibus, descriptionibus atque comentariis illustrantur. G.A. Lange, Berlin
Poss SG, Boschung HT (1996) Lancelets (Cephalochordata: Branchiostomatidae): how many species are valid? Isr J Zool 42(suppl):13–66
Pough FH, Janis CM et al (2005) Vertebrate life, 7th edn. Pearson Education, New Jersey
Roth VL (1988) The biological basis of homology. In: Humphries CJ (ed) Ontogeny and systematics. Columbia University Press, New York, pp 1–26
Sasai Y, Lu B et al (1994) Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell 79(5):779–790
Schubert M, Holland LZ et al (2000) Characterization of two amphioxus Wnt genes (AmphiWnt4 and AmphiWnt7b) with early expression in the developing central nervous system. Dev Dyn 217(2):205–215
Schubert M, Holland LZ et al (2001) Three amphioxus Wnt genes (AmphiWnt3, AmphiWnt5, and AmphiWnt6) associated with the tail bud: the evolution of somitogenesis in chordates. Dev Biol 240(1):262–273
Schubert M, Meulemans D et al (2003) Differential mesodermal expression of two amphioxus MyoD family members (AmphiMRF1 and AmphiMRF2). Gene Expr Patterns 3(2):199–202
Schubert M, Yu JK et al (2005) Retinoic acid signaling acts via Hox1 to establish the posterior limit of the pharynx in the chordate amphioxus. Development 1(132):61–73
Schubert M, Escriva H et al (2006) Amphioxus and tunicates as evolutionary model systems. Trends Ecol Evol 21(5):269–277
Scott IC, Steiglitz BM et al (2000) Spatiotemporal expression patterns of mammalian chordin during postgastrulation embryogenesis and in postnatal brain. Dev Dyn 217(4):449–456
Shimeld SM (1999) The evolution of the hedgehog gene family in chordates: insights from amphioxus hedgehog. Dev Genes Evol 209(1):40–47
Thompson JD, Gibson TJ et al (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25(24):4876–4882
Wagner GP (1989) The origin of morphological characters and the biological basis of homology. Evolution 43:1157–1171
Williams NA, Holland PWH (1996) Old head on young shoulders. Nature 383(6600):490
Yasui K, Zhang S et al (2000) Left–right asymmetric expression of BbPtx, a Ptx-related gene, in a lancelet species and the developmental left-sidedness in deuterostomes. Development 127(1):187–195
Yu JK, Holland LZ et al (2002) An amphioxus nodal gene (AmphiNodal) with early symmetrical expression in the organizer and mesoderm and later asymmetrical expression associated with left–right axis formation. Evol Dev 4(6):418–425
Yu JK, Satou Y et al (2007) Axial patterning in cephalochordates and the evolution of the organizer. Nature 445(7128):613–617
Acknowledgments
This work was funded by ANR and CNRS grants to HE. IS’ postdoctoral position was supported by a CNRS fellowship. SB’s postdoctoral position was supported by an EMBO long-term fellowship. We also thank two anonymous reviewers for their interesting comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.J. Gibson-Brown
Ildiko Somorjai and Stéphanie Bertrand contributed equally to the present work.
Rights and permissions
About this article
Cite this article
Somorjai, I., Bertrand, S., Camasses, A. et al. Evidence for stasis and not genetic piracy in developmental expression patterns of Branchiostoma lanceolatum and Branchiostoma floridae, two amphioxus species that have evolved independently over the course of 200 Myr. Dev Genes Evol 218, 703–713 (2008). https://doi.org/10.1007/s00427-008-0256-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-008-0256-6