Abstract
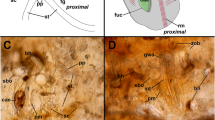
The digestive tract of Bilateria is a tube with a mouth at one end and an anus at the other end. Radiata, that include the phylum Cnidaria, have a blind-sac form of digestive tract with only one opening. It has therefore been commonly believed that the evolution of the body plan from Radiata to Bilateria included the change of the digestive tract from a blind sac to a tube. In this study, we report that there is a very narrow opening at the aboral end of hydra termed the aboral pore. This confirms a classical finding by Kanajew (Zool Anz, 76:37–44, 1928), but we confirmed it in both asexually reproduced and sexually reproduced polyps, demonstrating that the aboral pore represents innate morphology. We also find that the opening coincides with the site where synthesis of an extracellular matrix-degrading enzyme, hydra matrix metalloprotease, is elevated suggesting that the pore is maintained by extracellular matrix degradation. Finally, we find that there is material transfer through the opening in both inward and outward directions. From these observations, we conclude that the digestive tract and the body plan of hydra is not a blind sac as formerly believed but is a tube with a tapered end.



Similar content being viewed by others
References
Avery L, Horvitz HR (1989) Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of Caenorhabditis elegans. Neuron 3:473–485
Brusca RC, Brusca GJ, Nancy JH (1990) Invertebrates. Sinauer, Sunderland, MA, p 222
Campbell RD (1967) Tissue dynamics of steady state growth in Hydra littoralis. II. Patterns of tissue movement. J Morphol 121:19–28
Finnerty JR, Martindale MQ (1997) Homeoboxes in sea anemones (Cnidaria: Anthozoa): a PCR-based survey of Nematostella vectensis and Metridium senile. Biol Bull 193:62–76
Finnerty JR, Pang K, Burton P, Paulson D, Martindale MQ (2004) Origins of bilateral symmetry: Hox and dpp expression in a sea anemone. Science 304:1335–1337
Galliot B, Miller D (2000) Origin of anterior patterning. How old is our head? Trends Genet 16:1–5
Grens A, Gee L, Fisher DA, Bode HR (1996) CnNK-2, an NK-2 homeobox gene, has a role in patterning the basal end of the axis in hydria. Dev Biol 180:473–488
Haeckel E (1874) Die Gastrea Theorie, die phylogenetische Classification des Thierreiches und die Homologie der Keimblatter. Jena Z Naturwiss 8:1–55
Hyman L (1940) The invertebrates. McGraw-Hill, New York
Kanajew J (1928) Uber den Porus aboralis bei Pelmatohydra oligactis Pall. Zool Anz 76:37–44
LeLoup E (1962) Zooplankton Sheet 93. Conseil International Pour l’exploration de la mer, France
Leontovich AA, Zhang J, Shimokawa K, Nagase H, Sarras MP (2000) A novel hydra matrix metalloproteinase (HMMP) functions in extracellular matrix degradation, morphogenesis and the maintenance of differentiated cells in the foot process. Development 127:907–920
Lints TJ, Parsons LM, Hartley L, Lyons I, Harvey RP (1993) Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development 119:419–443
Main RJ (1928) Observations of the feeding mechanism of a ctenophore, Mnemiopsis leidyi. Biol Bull 55:69–78
Matus DQ, Pang K, Marlow H, Dunn CW, Thomsen GH, Martindale MQ (2006) Molecular evidence for deep evolutionary roots of bilaterality in animal development. Proc Natl Acad Sci USA 103:11195–11200
Meglitsch PA, Schram FR (1991) Invertebrate zoology. Oxford Univ. Press, New York
Meinhardt H (2002) The radial-symmetric hydra and the evolution of the bilateral body plan: an old body became a young brain. BioEssays 24:185–191
Okkema PG, Ha E, Haun C, Chen W, Fire A (1997) The Caenorhabditis elegans NK-2 homeobox gene ceh-22 activates pharyngeal muscle gene expression in combination with pha-1 and is required for normal pharyngeal development. Development 124:3965–3973
Ruppert EE, Barnes RD (1996) Invertebrate zoology. Saunders College, Fort Worth, pp 164–167
Sarras MP, Zhang X, Huff JK, Accavitti MA, St. John PL, Abrahamson DR (1993) Extracellular Matrix (mesoglea) of Hydra vulgaris. III. Formation and function during morphogenesis of Hydra cell aggregates. Dev Biol 157:383–398
Shimizu H, Zhang X, Zhang J, Leontovich A, Fei K, Yan L, Sarras MP Jr (2002) Epithelial morphogenesis in hydra requires de novo expression of extracellular matrix components and matrix metalloproteinases. Development 129:1521–1532
Shimizu H, Fujisawa T (2003) Peduncle of Hydra and the heart of higher organisms share a common ancestral origin. Genesis 36:182–186
Sugden AM, Jasny BR, Culotta E, Pennisi E (2003) Charting the evolutionary history of life. Science 300:1691
Sugiyama T, Fujisawa T (1978) Genetic analysis of developmental mechanisms in hydra. I. Sexual reproduction of Hydra magnipapillata and isolation of mutants. Dev Growth Differ 19:187–200
Acknowledgments
The authors thank Dr. M. Martindale at University of Hawaii for numerous suggestions, discussions, and information. This work was supported in part by a grant from Japanese ministry of education for HS (no. 60170191).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M.Q. Martindale
Electronic supplementary material
Below is the link to the electronic supplementary material.
Movie 1 Ingestion of India ink solution into the gastrovascular cavity through the aboral pore in floating polyps (MOV 389 kb)
Movie 2 Ejection of digested materials through the aboral pore (MOV 301 kb)
Rights and permissions
About this article
Cite this article
Shimizu, H., Takaku, Y., Zhang, X. et al. The aboral pore of hydra: evidence that the digestive tract of hydra is a tube not a sac. Dev Genes Evol 217, 563–568 (2007). https://doi.org/10.1007/s00427-007-0165-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-007-0165-0