Abstract
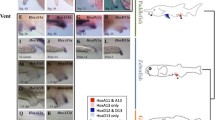
Hox genes form clusters. Invertebrates and Amphioxus have only one hox cluster, but in vertebrates, they are multiple, i.e., four in the basal teleost fish Polyodon and tetrapods (HoxA, B, C, D), but seven or eight in common teleosts. We earlier completely sequenced the entire hox gene loci in medaka fish, showing a total of 46 hox genes to be encoded in seven clusters (hoxAa, Ab, Ba, Bb, Ca, Da, Db). Among them, hoxAa, hoxAb and hoxDa clusters are presumed to be important for fin-to-limb evolution because of their key role in forelimb and pectoral fin development. In the present study, we compared genome organization and nucleotide sequences of the hoxAa and hoxAb clusters to these of tetrapod HoxA clusters, and found greater similarity in hoxAa case. We then analyzed expression of Abd-B family genes in the clusters. In the trunk, those from the hoxAa cluster, i.e., hoxA9a, hoxA10a, hoxA11a and hoxA13a, were expressed in a manner keeping the colinearity rule of the hox expression as those of tetrapods, while those from the hoxAb cluster, i.e., hoxA9b, hoxA10b, hoxA11b and hoxA13b, were not. In the pectoral fins, the hoxAa cluster was expressed in split domains and did not obey the rule. By contrast, those from the hoxAb and hoxDa clusters were expressed in a manner keeping the rule, i.e., an ancestral pattern similar to those of tetrapods. It is plausible that this differential expression of the two clusters is caused by changes occurred in global control regions after cluster duplications.





Similar content being viewed by others
References
Akimenko MA, Ekker M (1995) Anterior duplication of the Sonic hedgehog expression pattern in the pectoral fin buds of zebrafish treated with retinoic acid. Dev Biol 170:243–247
Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, Westerfield M, Ekker M, Postlethwait JH (1998) Zebrafish hox clusters and vertebrate genome evolution. Science 282:1711–1714
Amores A, Suzuki T, Yan YL, Pomeroy J, Singer A, Amemiya C, Postlethwait JH (2004) Developmental roles of pufferfish Hox clusters and genome evolution in ray-fin fish. Genome Res 14:1–10
Anand S, Wang WC, Powell DR, Bolanowski SA, Zhang J, Ledje C, Pawashe AB, Amemiya CT, Shashikant CS (2003) Divergence of Hoxc8 early enhancer parallels diverged axial morphologies between mammals and fishes. Proc Natl Acad Sci USA 100:15666–15669
Belting HG, Shashikant CS, Ruddle FH (1998) Modification of expression and cis-regulation of Hoxc8 in the evolution of diverged axial morphology. Proc Natl Acad Sci USA 95:2355–2360
Bruce AE, Oates AC, Prince VE, Ho RK (2001) Additional hox clusters in the zebrafish: divergent expression patterns belie equivalent activities of duplicate hoxB5 genes. Evol Dev 3:127–144
Burke AC, Nelson CE, Morgan BA, Tabin C (1995) Hox genes and the evolution of vertebrate axial morphology. Development 121:333–346
Charité J, McFadden DG, Olson EN (2000) The bHLH transcription factor dHAND controls Sonic hedgehog expression and establishment of the zone of polarizing activity during limb development. Development 127:2461–2470
Dietrich S, Abou-Rebyeh F, Brohmann H, Bladt F, Sonnenberg-Riethmacher E, Yamaai T, Lumsden A, Brand-Saberi B, Birchmeier C (1999) The role of SF/HGF and c-Met in the development of skeletal muscle. Development 126:1621–1629
Dollé P, Izpisúa-Belmonte JC, Falkenstein H, Renucci A, Duboule D (1989) Coordinate expression of the murine Hox-5 complex homeobox-containing genes during limb pattern formation. Nature 342:767–772
Duboule D, Dollé P (1989) The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. EMBO J 8:1497–1505
Ferrier DE, Minguillón C, Holland PW, Garcia-Fernàndez J (2000) The amphioxus Hox cluster: deuterostome posterior flexibility and Hox14. Evol Dev 2:284–293
Gehring WJ, Affolter M, Bürglin T (1994) Homeodomain proteins. Annu Rev Biochem 63:487–526
Gérard M, Duboule D, Zákány J (1993) Structure and activity of regulatory elements involved in the activation of the Hoxd-11 gene during late gastrulation. EMBO J 12:3539–3550
Haines L, Neyt C, Gautier P, Keenan DG, Bryson-Richardson RJ, Hollway GE, Cole NJ, Currie PD (2004) Met and Hgf signaling controls hypaxial muscle and lateral line development in the zebrafish. Development 131:4857–4869
Harding K, McGinnis W, Wedeen C, Levine M (1985) Spatially regulated expression of homeotic genes in Drosophila. Science 229:1236–1242
Hart CP, Fainsod A, Ruddle FH (1987) Sequence analysis of the murine Hox-2.2, -2.3 and -2.4 homeo boxes: evolutionary and structural comparisons. Genomics 1:182–195
Holland PW (1999) Gene duplication: past, present and future. Semin Cell Dev Biol 10:541–547
Holland PW, Garcia-Fernàndez J (1996) Hox genes and chordate evolution. Dev Biol 173:382–395
Inohaya K, Yasumasu S, Ishimaru M, Ohyama A, Iuchi I, Yamagami K (1995) Temporal and spatial patterns of gene expression for the hatching enzyme in the teleost embryo, Oryzias latipes. Dev Biol 171:374–385
Inohaya K, Yasumasu S, Yasumasu I, Iuchi I, Yamagami K (1999) Analysis of the origin and development of hatching gland cells by transplantation of the embryonic shield in the fish, Oryzias latipes. Dev Growth Differ 41:557–566
Iwamatsu T (2004) Stages of normal development in the medaka Oryzias latipes. Mech Dev 121:605–618
Izpisúa-Belmonte JC, Falkenstein H, Dollé P, Renucci A, Duboule D (1991) Murine genes related to the Drosophila Abd-B homeotic genes are sequentially expressed during development of the posterior part of the body. EMBO J 10:2279–2289
Kessel M, Gruss P (1990) Murine development control genes. Science 249:374–379
Krumlauf R (1992) Evolution of the vertebrate Hox homeobox genes. Bioessays 14:245–252
Kurosawa G, Yamada K, Ishiguro H, Hori H (1999) Hox gene complexity in medaka fish may be similar to that in pufferfish rather than zebrafish. Biochem Biophys Res Commun 260:66–70
Kurosawa G, Takamatsu N, Takahashi M, Sumitomo M, Sanaka M, Yamada K, Nishii K, Matsuda M, Asakawa S, Ishiguro H, Miura K, Kurosawa Y, Shimizu N, Kohara Y, Hori H (2006) Organization and structure of hox gene loci in medaka genome and comparison with those of pufferfish and zebrafish genomes. Gene 370:75–82
Levine M, Hafen E, Garber RL, Gehring WJ (1983) Spatial distribution of Antennapedia transcripts during Drosophila development. EMBO J 2:2037–2046
Metscher BD, Takahashi K, Crow K, Amemiya C, Nonaka DF, Wagner DP (2005) Expression of Hoxa-11 and Hoxa-13 in the pectoral fin of a basal ray-finned fish, Polyodon spathula: implications for the origin of tetrapod limbs. Evol Dev 7:186–195
Naruse K, Fukamachi S, Mitani H, Kondo M, Matsuoka T, Kondo S, Hanamura N, Morita Y, Hasegawa K, Nishigaki R, Shimada A, Wada H, Kusakabe T, Suzuki N, Kinoshita M, Kanamori A, Terado T, Kimura H, Nonaka M, Shima A (2000) A detailed linkage map of medaka, Oryzias latipes: comparative genomics and genome evolution. Genetics 154:1773–1784
Neumann CJ, Grandel H, Gaffield W, Schulte-Merker F, Nüsslein-Volhard C (1999) Transient establishment of anterior–posterior polarity in the zebrafish pectoral fin bud in the absence of sonic hedgehog activity. Development 126:4817–4826
Neyt C, Jagla K, Thisse C, Thisse B, Haines L, Currie PD (2000) Evolutionary origins of vertebrate appendicular muscle. Nature 408:82–86
Prince VE, Joly L, Ekker M, Ho RK (1998) Zebrafish hox genes: genomic organization and modified collinear expression patterns in the trunk. Development 125:407–420
Riddle RD, Johnson RL, Laufer E, Tabin C (1993) Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 75:1401–1416
Santini S, Boore JL, Meyer A (2003) Evolutionary conservation of regulatory elements in vertebrate Hox gene clusters. Genome Res 13:1111–1122
Schwartz S, Zhang Z, Frazer KA, Smit A, Riemer C, Bouck J, Gibbs R, Hardison R, Miller W (2000) PipMaker—a web server for aligning two genomic DNA sequences. Genome Res 10:577–586
Scott MP (1992) Vertebrate homeobox gene nomenclature. Cell 71:551–553
Sordino P, van der Hoeven F, Duboule D (1995) Hox gene expression in teleost fins and the origin of vertebrate digits. Nature 375:678–681
Sordino P, Duboule D, Kondo T (1996) Zebrafish Hoxa and Evx-2 genes: cloning, developmental expression and implication for the functional evolution of posterior Hox genes. Mech Dev 59:165–175
Spitz F, Gonzalez F, Duboule D (2003) A global control region defines a chromosomal regulatory landscape containing the HoxD cluster. Cell 113:405–417
Tümpel S, Cambronero F, Widedemann LM, Krumlauf R (2006) Evolution of cis element in the differential expression of two Hoxa2 coparalogous genes in pufferfish (Takifugu rubripes). Proc Natl Acad Sci USA 103:5419–5424
van der Hoeven F, Sordino P, Fraudeau N (1996a) Teleost HoxD and HoxA genes: comparison with tetrapods and functional evolution of the HOXD complex. Mech Dev 54:9–21
van der Hoeven F, Zákány J, Duboule D (1996b) Gene transpositions in the HoxD complex reveal a hierarchy of regulatory controls. Cell 85:1025–1035
Yamomoto M, Gotoh Y, Tamura K, Tanaka M, Kawakami A, Ide H, Kuroiwa A (1998) Coordinated expression of Hoxa-11 and Hoxa-13 during limb muscle patterning. Development 125:1325–1335
Yokouchi Y, Sasaki H, Kuroiwa A (1991) Homeobox gene expression correlated with the bifurcation process of limb cartilage development. Nature 353:443–445
Acknowledgement
We thank Dr. Keiji Inohaya for the instruction regarding the medaka in situ method, Susumu Hamada, Makiko Tsutsumi, and Rieko Yamamoto for their help in manipulating the medaka Hd–rR BAC library. We also thank Dr. Johannes Martinus Dijkstra for helpful discussions. This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science, and Technology in Japan and by Grant-in-Aid for Special Project Research to H.H. (No. 12202004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Hollemann
Rights and permissions
About this article
Cite this article
Takamatsu, N., Kurosawa, G., Takahashi, M. et al. Duplicated Abd-B class genes in medaka hoxAa and hoxAb clusters exhibit differential expression patterns in pectoral fin buds. Dev Genes Evol 217, 263–273 (2007). https://doi.org/10.1007/s00427-007-0137-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-007-0137-4