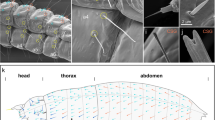
Abstract
Small chemosensory proteins (CSPs) belong to a conserved, but poorly understood, protein family found in insects and other arthropods. They exhibit both broad and restricted expression patterns during development. In this paper, we used a combination of genome annotation, transcriptional profiling and RNA interference to unravel the functional significance of a honeybee gene (csp5) belonging to the CSP family. We show that csp5 expression resembles the maternal-zygotic pattern that is characterized by the initiation of transcription in the ovary and the replacement of maternal mRNA with embryonic mRNA. Blocking the embryonic expression of csp5 with double-stranded RNA causes abnormalities in all body parts where csp5 is highly expressed. The treated embryos show a “diffuse”, often grotesque morphology, and the head skeleton appears to be severely affected. They are ‘unable-to-hatch’ and cannot progress to the larval stages. Our findings reveal a novel, essential role for this gene family and suggest that csp5 (unable-to-hatch) is an ectodermal gene involved in embryonic integument formation. Our study confirms the utility of an RNAi approach to functional characterization of novel developmental genes uncovered by the honeybee genome project and provides a starting point for further studies on embryonic integument formation in this insect.




Similar content being viewed by others
References
Ban L, Scaloni A, Brandazza A, Angeli S, Zhang L, Yan Y, Pelosi P (2003) Chemosensory proteins of Locusta migratoria. Insect Mol Biol 12:125–134
Beye M, Hartel S, Hagen A, Hasselmann M, Omholt SW (2002) Specific developmental gene silencing in the honey bee using a homeobox motif. Insect Mol Biol 11:527–532
Beye M, Hasselmann M, Fondrk MK, Page RE, Omholt SW (2003) The gene csd is the primary signal for sexual development in the honeybee and encodes an SR-type protein. Cell 114:419–429
Bicker G (1995) Transmitter-induced calcium signalling in cultured neurons of the insect brain. J Neurosci Methods 69(1):33–41
DuPraw EJ (1961) A unique hatching process in the honeybee. Trans Am Microsc Soc 80:185–191
Forêt S, Maleszka R (2006) Function and evolution of a gene family encoding odorant binding-like proteins in a social insect, the honey bee (Apis mellifera). Genome Res 16:1401–1413
Forêt S, Wanner KW, Maleszka R (2006) Chemosensory proteins in the honey bee: insights from the annotated genome, comparative analyses and expressional profiling. Insect Biochem Mol Biol 37:19–28
Honey Bee Genome Consortium (2006) Insights into social insects from the genome of the honey bee Apis mellifera. Nature 443:931–949
Juarez P (1994) Hydrocarbon biosynthesis in Triatoma infestans eggs. Arch Insect Biochem Physiol 25:193–206
Jung-Offmann I (1968) Traité de biologie de l’abeille, chapter Développement et croissance. Masson, pp 69–144
King RC (1985) Comprehensive insect physiology, biochemistry and pharmacology, vol 1, chapter The origin and functioning of insect oocytes and nurse cells. Pergamon, pp 37–82
Kitabayashi AN, Arai T, Kubo T, Natori S (1998) Molecular cloning of cDNA for p10, a novel protein that increases in the regenerating legs of Periplaneta americana (American cockroach). Insect Biochem Mol Biol 28:785–790
Kucharski R, Ball EE, Hayward DC, Maleszka R (2000) Molecular cloning and expression analysis of a cDNA encoding a glutamate transporter in the honeybee brain. Gene 242:399–405
Kucharski R, Maleszka R (2002a) Molecular profiling of behavioural development: differential expression of mRNAs for inositol 1,4,5-trisphosphate 3-kinase isoforms in naive and experienced honeybees (Apis mellifera). Mol Brain Res 99:92–101
Kucharski R, Maleszka R (2002b) Evaluation of differential gene expression during behavioral development in the honeybee using microarrays and Northern blots. Genome Biol 3:7.1–7.9
Maleszka R, Helliwell P, Kucharski R (2000) Pharmacological interference with glutamate re-uptake impairs long-term memory in the honeybee, Apis mellifera. Behav Brain Res 115:49–53
Marchese S, Angeli S, Andolfo A, Scaloni A, Brandazza A, Mazza M, Picimbon J, Leal WS, Pelosi P (2000) Soluble proteins from chemosensory organs of Eurycantha calcarata (Insects, Phasmatodea). Insect Biochem Mol Biol 30:1091–1098
Miklos GL, Maleszka R (2001) Protein functions and biological contexts. Proteomics 1(2):169–178
Nelson JA (1915) The embryology of the honeybee. Princeton University Press
Osborne P, Dearden PK (2005) Non-radioactive in-situ hybridization to honeybee embryos and ovaries. Apidologie 36:113–118
Ostrowski S, Dierick HA, Bejsovec A (2002) Genetic control of cuticle formation during embryonic development of Drosophila melanogaster. Genetics 161:171–182
Pelosi P, Calvello M, Ban L (2005) Diversity of odorant-binding proteins and chemosensory proteins in insects. Chem Senses 30(Suppl 1):i291–i292
Robinson GE, Evans JD, Maleszka R, Robertson HM, Weaver DB, Worley K, Gibbs RA, Weinstock GM (2006) Sweetness and light: illuminating the honey bee genome. Insect Mol Biol 15:535–539
Schal C, Sevala V, Young HP, Bachmann JAS (1998) Sites of synthesis and transport pathways of insect hydrocarbons: cuticle and ovary as target tissues. Am Zool 38:382–393
Tadros W, Houston SA, Bashirullah A, Cooperstock RL, Semotok JL, Reed BH, Lipshitz HD (2003) Regulation of maternal transcript destabilization during egg activation in Drosophila. Genetics 164:989–1001
Truman JW, Riddiford LM (2002) Endocrine insights into the evolution of metamorphosis in insects. Annu Rev Entomol 47:467–500
Vogt RG (2004) Molecular basis of pheromone detection in insects. In: Gilbert LI, Iatro K, Gill S (eds) Comprehensive molecular insect science, vol 3. Elsevier, pp 753–803
Wang T, Jorda M, Jones PL, Maleszka R, Ling X, Robertson HM, Mizzen CA, Peinado M, Robinson GE (2006) Functional CpG methylation system in a social insect. Science 314(5799):645–647
Wanner KW, Willis LG, Theilmann DA, Isman MB, Feng Q, Plettner E (2004) Analysis of the insect os-d-like gene family. J Chem Ecol 30:889–911
Wanner KW, Isman MB, Feng Q, Plettner E, Theilmann DA (2005) Developmental expression patterns of four chemosensory protein genes from the Eastern spruce budworm, Chroistoneura fumiferana. Insect Mol Biol 14 (3):289–300
Acknowledgement
Martin Beye, Martin Hassleman and Eldon Ball provided advice and protocols for which we are grateful. We also thank Paul Helliwell for mastering a ‘perfect’ injecting technique and for excellent bee keeping.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Roth
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1
RT-PCR amplification of uth and Dnmt in 48-h-old honeybee embryos injected at 10 ± 2 h with dsRNA (+) and in control eggs (−) injected with DNA. The primers for Dnmt are described in Wang et al. (2006). Computer-generated images (MD Phosphor-Imager 400S) of agarose gels were analysed using ImageQuant software (Kucharski and Maleszka 2002a, b). The levels of both genes in dsRNA-treated embryos are shown relatively to controls (100%) (JPG 593 kb).
Fig. S2
In situ hybridization showing the expression of csp5/uth in 44- to 48-old embryos: a Longitudinal sections plus DAPI staining, b transverse section through thoracic region showing the form and relation of the ectoderm (E) and mesoderm (M). Mesoderm is composed of two layers continuous with one another at the lateral margins (Nelson 1915) (JPG 805 kb).
Fig. S3
In situ hybridization of uth showing strong labelling in the cerebral lobes of 66- to 70-h-old embryo. See Fig. 3 for more details (JPG 1.16 kb).
Fig. S4
a Black arrows show the cuticular structures maintaining the shape of the control embryo. b–d dsRNA-injected embryos lack the cuticular scaffolding. In all embryos, the chorion is indicated by white arrows (JPG 6.20 kb).
Rights and permissions
About this article
Cite this article
Maleszka, J., Forêt, S., Saint, R. et al. RNAi-induced phenotypes suggest a novel role for a chemosensory protein CSP5 in the development of embryonic integument in the honeybee (Apis mellifera) . Dev Genes Evol 217, 189–196 (2007). https://doi.org/10.1007/s00427-006-0127-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-006-0127-y