Abstract
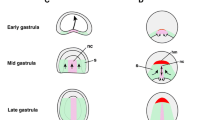
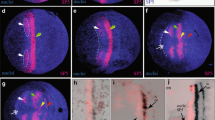
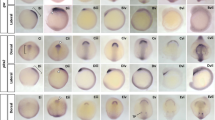
In this paper, we define temporal and spatial subdivisions of the embryonic head mesoderm and describe the fate of the main lineages derived from this tissue. During gastrulation, only a fraction of the head mesoderm (primary head mesoderm; PHM) invaginates as the anterior part of the ventral furrow. The PHM can be subdivided into four linearly arranged domains, based on the expression of different combinations of genetic markers (tinman, heartless, snail, serpent, mef-2, zfh-1). The anterior domain (PHMA) produces a variety of cell types, among them the neuroendocrine gland (corpus cardiacum). PHMB, forming much of the “T-bar” of the ventral furrow, migrates anteriorly and dorsally and gives rise to the dorsal pharyngeal musculature. PHMC is located behind the T-bar and forms part of the anterior endoderm, besides contributing to hemocytes. The most posterior domain, PHMD, belongs to the anterior gnathal segments and gives rise to a few somatic muscles, but also to hemocytes. The procephalic region flanking the ventral furrow also contributes to head mesoderm (secondary head mesoderm, SHM) that segregates from the surface after the ventral furrow has invaginated, indicating that gastrulation in the procephalon is much more protracted than in the trunk. We distinguish between an early SHM (eSHM) that is located on either side of the anterior endoderm and is the major source of hemocytes, including crystal cells. The eSHM is followed by the late SHM (lSHM), which consists of an anterior and posterior component (lSHMa, lSHMp). The lSHMa, flanking the stomodeum anteriorly and laterally, produces the visceral musculature of the esophagus, as well as a population of tinman-positive cells that we interpret as a rudimentary cephalic aorta (“cephalic vascular rudiment”). The lSHM contributes hemocytes, as well as the nephrocytes forming the subesophageal body, also called garland cells.









Similar content being viewed by others
References
Anderson DT (1962) The embryology of Dacus tyroni (Frogg) (Diptera, Trypetidae (Tephritidae)), the Queensland fruit-fly. J Embryol Exp Morph 10:248–292
Arendt D, Nuebler-Jung K (1996) Common ground plans in early brain development in mice and flies. Bioessays 18:255–259
Asburner M (1989) Drosophila. A laboratory manual. Cold Spring Harbor Press, Cold Spring Harbor, NY
Beiman M, Shilo BZ, Volk T (1996) Heartless, a Drosophila FGF receptor homolog, is essential for cell migration and establishment of several mesodermal lineages. Genes Dev 10:2993–3002
Borkowski OM, Brown NH, Bate M (1995) Anterior–posterior subdivision and the diversification of the mesoderm in Drosophila. Development 121:4183–4193
Boyan GS, Williams JLD, Posser S, Bräunig P (2002) Morphological and molecular data argue for the labrum being non-apical, articulated, and the appendage of the intercalary segment in the locust. Arthropod Struct Develop 31:65–76
Campos-Ortega JA, Hartenstein V (1997) The embryonic development of Drosophila melanogaster, 2nd edn. Springer, Berlin Heidelberg New York
Chang T, Mazotta J, Dumstrei K, Dumitrescu A, Hartenstein V (2001) Dpp and Hh signaling in the Drosophila embryonic eye field. Development 128:4691–4704
Chapman RF (1982) The insects: structure and function. Harvard University Press, Cambridge, MA
Chen JN, Fishman MC (2000) Genetics of heart development. Trends Genet 16:383–388
Couly GF, Coltey PM, Le Douarin NM (1992) The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development 114:1–15
Crossley AC (1985). Nephrocytes and pericardial cells. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry, and pharmacology, vol 3. Pergamon, Oxford
Denholm B, Sudarsan V, Pasalodos-Sanchez S, Artero R, Lawrence P, Maddrell S, Baylies M, Skaer H (2003) Dual origin of the renal tubules in Drosophila: mesodermal cells integrate and polarize to establish secretory function. Curr Biol 13:1052–1057
De Robertis EM, Kuroda H (2004) Dorsal–ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol 20:285–308
De Robertis EM, Sasai Y (1996) A common plan for dorso-ventral patterning in Bilateria. Nature 380:37–40
Diederich RJ, Pattatucci AM, Kaufman TC (1991) Developmental and evolutionary implications of labial, Deformed and engrailed expression in the Drosophila head. Development 113:273–281
De Velasco B, Shen J, Go S, Hartenstein V (2004) Embryonic development of the Drosophila corpus cardiacum, a neuroendocrine gland with similarity to the vertebrate pituitary, is controlled by sine oculis and glass. Dev Biol 274:280–294
Eastham LES (1930a) The formation of germ layers in insects. Biol Rev 5:1–29
Eastham LES (1930b) The embryology of Pieris rapae. Organogeny. Phil Trans Roy Soc Lond B 219:1–50
Evans CJ, Hartenstien V, Benerjee U (2003) Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev Cell 5:673–690
Foe V (1989) Mitotic domains reveal early commitment of cells in Drosophila embryos. Development 107:1–22
Francis-West PH, Robson L, Evans DJ (2003) Craniofacial development: the tissue and molecular interactions that control development of the head. Adv Anat Embryol Cell Biol 169:1–138
Gormley JP, Nascone-Yoder NM (2003) Left and right contributions to the Xenopus heart: implications for asymmetric morphogenesis. Dev Genes Evol 213:390–398
Haas MS, Brown SJ, Beeman RW (2001) Pondering the procephalon: the segmental origin of the labrum. Dev Genes Evol 211:89–95
Herbomel P, Thisse B, Thisse C (1999) Ontogeny and behavior of early macrophages in the zebrafish embryo. Development 126:3735–3745
Heymons R (1895) Die Embryon alentwicklung von Dermapteren und Orthopteren unter besonderer Beruecksichtigung der Keimblaetterbildung. Gustav Fischer, Jena, pp 1–136
Holley SA, Ferguson EL (1997) Fish are like flies are like frogs: conservation of dorsal–ventral patterning mechanisms. Bioessays 19:281–284
Inamdar MS (2003) Drosophila asrij is expressed in pole cells, trachea and hemocytes. Dev Genes Evol 213:134–137
Lebestky T, Chang T, Hartenstein V, Banerjee U (2000) Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science 288:146–149
Mandal L, Banerjee U, Hartenstein V (2004). Evidence for a hemangioblast and similarities between lymph gland hematopoiesis in Drosophila and mammalian AGM. Nat Genet 36:1019–1023
Meier SP (1982) The development of segmentation in the cranial region of vertebrate embryos. Scan Electron Microsc (Pt 3):1269–1282
Mohun T, Orford R, Shang C (2003) The origins of cardiac tissue in the amphibian, Xenopus laevis. Trends Cardiovasc Med 13:244–248
Moore AW, Barbel S, Jan LY, Jan YN (2000) A genomewide survey of basic helix-loop-helix factors in Drosophila. Proc Natl Acad Sci U S A 97:10436–10441
Nardi JB (2004) Embryonic origins of the two main classes of hemocytes—granular cells and plasmatocytes—in Manduca sexta. Dev Genes Evol 214:19–28
Nardi JB, Pilas B, Ujhelyi E, Garsha K, Kanost MR (2003) Hematopoietic organs of Manduca sexta and hemocyte lineages. Dev Genes Evol 213:477–491
Nguyen HT, Bodmer R, Abmayr SM, McDermott JC, Spoerel NA (1994) D-mef2: a Drosophila mesoderm-specific MADS box-containing gene with a biphasic expression profile during embryogenesis. Proc Natl Acad Sci U S A 91:7520–7524
Paterson NF (1932) A contribution to the embryological development of Euryope terminalis. Pt II. Organogeny. S Afr J Sci 29:414–448
Noden DM (1988) Interactions and fates of avian craniofacial mesenchyme. Development 103:121–140
Poulson DF (1950) Histogenesis, organogenesis, and differentiation in the embryo of Drosophila melanogaster (Meigen). In: Demerec M (ed) Biology of Drosophila. Wiley, New York, pp 168–274
Rehorn KP, Thelen H, Michelson AM, Reuter R (1996) A molecular aspect of hematopoiesis and endoderm development common to vertebrates and Drosophila. Development 122:4023–4031
Riechmann V, Irion U, Wilson R, Grosskortenhaus R, Leptin M (1997) Control of cell fates and segmentation in the Drosophila mesoderm. Development 124:2915–2922
Rogers BT, Kaufman TC (1997) Structure of the insect head in ontogeny and phylogeny: a view from Drosophila. Int Rev Cytol 174:1–84
Rohrschneider I (1968) Beitraege zur Entwicklung des Vorderkopfes und der Mundregion von Periplaneta americana. Zool Jahrb Abt Anat Ontog Tiere 85:537–578
Roonwal ML (1937) Studies on the embryology of the African migratory locust, Locusta migratoria migratorioides. II. Organogeny. Phil Trans Roy Soc Lond B 227:175–244
Schneider MD, Gaussin V, Lyons KM (2003) Tempting fate: BMP signals for cardiac morphogenesis. Cytokine Growth Factor Rev 14:1–4
Seecoomar M, Agarwal S, Vani K, Yang G, Mohler J (2000) Knot is required for the hypopharyngeal lobe and its derivatives in the Drosophila embryo. Mech Dev 91:209–215
Snodgrass RE (1935) Principles of insect morphology. McGraw-Hill, New York, pp 311–315
Staehling-Hampton K, Hoffmann FM, Baylies MK, Rushton E, Bate M (1994) dpp induces mesodermal gene expression in Drosophila. Nature 372:783–786
Strindberg H (1913) Embryologische Studien an Insekten. Zeit F Wiss Zool 106:1–227
Teichmann U, Kessel M (2004) Highly restricted BMP10 expression in the trabeculating myocardium of the chick embryo. Dev Genes Evol 214:96–98
Tepass U, Fessler L, Aziz A, Hartenstein V (1994) Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development 120:1829–1837
Tiegs OW, Murray FV (1938) The embryonic development of Calandra oryzae. Quart J Microscop Sci 80:159–271
Tomancak P, Beaton A, Weiszmann R, Kwan E, Shu SQ, Lewis S, Richards S, Ashburner M, Hartenstein V, Celniker SE, Rubin GM (2002) Systematic determination of patterns of gene expression during Drosophila embryogenesis. Genome Biol 3:1–18; on the web at http://www.genomebiology.com/2002/3/12/research/0088
Tzahor E, Kempf H, Mootoosamy RC, Poon AC, Abzhanov A, Tabin CJ, Dietrich S, Lassar AB (2003) Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes Dev 17:3087–3099
Ullmann SL (1964) The origin and structure of the mesoderm and the formation of the coelomic sacs in Tenebrio molitor L (Insecta, Coleoptera). Phil Trans Roy Soc Lond B 747:245–276
Wachtler F, Jacob M (1986) Origin and development of the cranial skeletal muscles. Bibl Anat 29:24–46
Ward EJ, Skeath JB (2000) Characterization of a novel subset of cardiac cells and their progenitors in the Drosophila embryo. Development 22:4959–4969
Weinstein DC, Hemmati-Brivanlou A (1999) Neural induction. Annu Rev Cell Dev Biol 15:411–433
Wheeler WM (1893) A contribution to insect embryology. J Morph 8:1–160
Yin Z, Xu XL, Frasch M (1997) Regulation of the twist target gene tinman by modular cis-regulatory elements during early mesoderm development. Dev 124:4971–4982
Acknowledgements
We thank Drs. M. Frasch , T. Tabata, R. Renkawitz-Pohl, Z.C. Lai, H.T. Nguyen, R. White , A.M. Michelson, U. Banerjee, and J. Lengyel for providing us the fly stocks, cDNA, and antibodies. We would also like to thank the Bloomington Stock Center for fly stocks and the Berkeley Drosophila Genome Project for some of the figures. This work was supported by a UCLA Dissertation Fellowship to B.d.V. and NIH grant NS29367 to V.H.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Roth
Rights and permissions
About this article
Cite this article
de Velasco, B., Mandal, L., Mkrtchyan, M. et al. Subdivision and developmental fate of the head mesoderm in Drosophila melanogaster . Dev Genes Evol 216, 39–51 (2006). https://doi.org/10.1007/s00427-005-0029-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-005-0029-4