Abstract
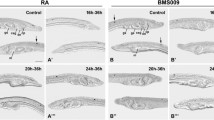
Retinoic acid (RA) signaling plays critical roles in the regionalization of the central nervous system and mesoderm of all vertebrates that have been examined. However, to date, a role for RA in pancreas and liver development has only been demonstrated for the teleost zebrafish. Here, we demonstrate that RA signaling is required for development of the pancreas but not the liver in the amphibian Xenopus laevis and the avian quail. We disrupted RA signaling in Xenopus tadpoles, using both a pharmacological and a dominant-negative strategy. RA-deficient quail embryos were obtained from hens with a dietary deficiency in vitamin A. In both species we found that pancreas development was dependent on RA signaling. Furthermore, treatment of Xenopus tadpoles with exogenous RA led to an expansion of the pancreatic field. By contrast, liver development was not perturbed by manipulation of RA signaling. Taken together with our previous finding that RA signaling is necessary and sufficient for zebrafish pancreas development, these data support the hypothesis that a critical role for RA signaling in pancreas development is a conserved feature of the vertebrates.






Similar content being viewed by others
References
Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H (1998) Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev 12:1763–1768
Apelqvist A, Ahlgren U, Edlund H (1997) Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr Biol 7:801–804
Argenton F, Zecchin E, Bortolussi M (1999) Early appearance of pancreatic hormone-expressing cells in the zebrafish embryo. Mech Dev 87:217–221
Begemann G, Schilling TF, Rauch GJ, Geisler R, Ingham PW (2001) The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development 128: 3081–3094
Biemar F, Argenton F, Schmidtke R, Epperlein S, Peers B, Driever W (2001) Pancreas development in zebrafish: early dispersed appearance of endocrine hormone expressing cells and their convergence to form the definitive islet. Dev Biol 230:189–203
Costa RM, Mason J, Lee M, Amaya E, Zorn AM (2003) Novel gene expression domains reveal early patterning of the Xenopus endoderm. Gene Expr Patterns 3:509–519
Deutsch G, Jung J, Zheng M, Lora J, Zaret KS (2001) A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development 128:871–881
Ericson J, Thor S, Edlund T, Jessell TM, Yamada T (1992) Early stages of motor neuron differentiation revealed by expression of homeobox gene Islet-1. Science 256:1555–1560
Gale E, Zile M, Maden M (1999) Hindbrain respecification in the retinoid-deficient quail. Mech Dev 89:43–54
Germain P, Iyer J, Zechel C, Gronemeyer H (2002) Co-regulator recruitment and the mechanism of retinoic acid receptor synergy. Nature 415:187–92
Grandel H, Lun K, Rauch GJ, Rhinn M, Piotrowski T, Houart C, Sordino P, Kuchler AM, Schulte-Merker S, Geisler R et al (2002) Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development 129:2851–2865
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–92
Hebrok M, Kim SK, Melton DA (1998) Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev 12:1705–1713
Horb ME, Slack JM (2002) Expression of amylase and other pancreatic genes in Xenopus. Mech Dev 113:153–157
Kelly OG, Melton DA (2000) Development of the Pancreas in Xenopus laevis. Dev Dyn 218:615–627
Kim SK, Hebrok M, Melton DA (1997a) Notochord to endoderm signaling is required for pancreas development. Development 124:4243–4252
Kim SK, Hebrok M, Melton DA (1997b) Pancreas development in the chick embryo. Cold Spring Harb Symp Quant Biol 62:377–383
Kumar M, Jordan N, Melton DA, Grapin-Botton A (2003) Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev Biol 259:109–122
Leise WF III, Mueller PR (2002) Multiple Cdk1 inhibitory kinases regulate the cell cycle during development. Dev Biol 249:156–173
Maden M, Gale E, Kostetskii I, Zile M (1996) vitamin A-deficient quail embryos have half a hindbrain and other neural defects. Curr Biol 6:417–426
Milewski WM, Duguay SJ, Chan SJ, Steiner DF (1998) Conservation of PDX-1 structure, function, and expression in zebrafish. Endocrinology 139:1440–1449
Moriya N, Komazaki S, Asashima M (2000) In vitro organogenesis of pancreas in Xenopus laevis dorsal lips treated with retinoic acid. Dev Growth Differ 42:175–185
Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dolle P (2002) Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse. Development 129:3563–3574
Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dollé P (2000) Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development 127:75–85
Nieuwkoop PD, Faber J (1994) Normal table of Xenopus laevis (Daudin): a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. Garland, New York
Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV (1996) PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 122:983–995
Ohlsson H, Karlsson K, Edlund T (1993) IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J 12:4251–4259
Quinlan R, Gale E, Maden M, Graham A (2002) Deficits in the posterior pharyngeal endoderm in the absence of retinoids. Dev Dyn:54–60
Roy S, Qiao T, Wolff C, Ingham P (2001) Hedgehog signalling pathway is essential for pancreas specification in the zebrafish embryo. Curr Biol 11:1358–1363
Sanders TA, Lumsden A, Ragsdale CW (2002) Arcuate plan of chick midbrain development. J Neurosci 24:10742–10750
Sharpe CR, Goldstone K (1997) Retinoid receptors promote primary neurogenesis in Xenopus. Development 124:515–523
Sive HL, Grainger RM, Harland RM (2000) Early development of Xenopus laevis: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor
Sparrow DB, Cai C, Kotecha S, Latinkic B, Cooper B, Towers N, Evans SM, Mohun TJ (2000) Regulation of the tinman homologues in Xenopus embryos. Dev Biol 227:65–79
Stafford D, Prince VE (2002) Retinoid signaling is required for a critical early step in zebrafish pancreatic development. Curr Biol 12:1–20
Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF (1997) Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet 15:106–110
Thomas MK, Rastalsky N, Lee JH, Habener JF (2000) Hedgehog signaling regulation of insulin production by pancreatic beta-cells. Diabetes 49:2039–4207
Yatskievych TA, Pascoe S, Antin PB (1999) Expression of the homeobox gene Hex during early stages of chick embryo development. Mech Dev 80:107–109
Yee NS, Yusuff S, Pack M (2001) Zebrafish pdx1 morphant displays defects in pancreas development and digestive organ chirality, and potentially identifies a multipotent pancreas progenitor cell. Genesis 30:137–140.
Zeynali B, Dixon KE (1998) Effects of retinoic acid on the endoderm in Xenopus embryos. Dev Genes Evol 208:318–326
Zorn AM, Mason J (2001) Gene expression in the embryonic Xenopus liver. Mech Dev 103:153–157
Acknowledgements
We are indebted to Aaron Zorn, Rob Baker, and Maya Kumar for supplying probes. We also thank Aaron Zorn for comments on the manuscript. We are grateful to Hazel Sive for supplying DN-RAR constructs, and to Emily Gale for supplying the VAD quail embryos from the breeding colony at King’s College London, funded by BBSRC. This work was supported by NIH grant No. RO1 DK064973-01 and JDRF grant No. 1-2003-257 to VEP, and NIH grant No. RO1 CA84007 to PRM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by R.P. Elinson
Rights and permissions
About this article
Cite this article
Stafford, D., Hornbruch, A., Mueller, P.R. et al. A conserved role for retinoid signaling in vertebrate pancreas development. Dev Genes Evol 214, 432–441 (2004). https://doi.org/10.1007/s00427-004-0420-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-004-0420-6