Abstract
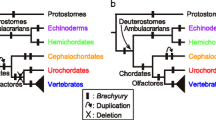
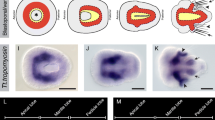
Trichoplax adhaerens is the only species known from the phylum Placozoa with one of the simplest metazoan body plans. In the small disc-like organism an upper and a lower epithelium can be distinguished with a less compact third cell layer in between. When Trichoplax was first described in 1883, the relation of these three cell layers with ectoderm, endoderm and mesoderm of higher animals was discussed. Still, little is known about embryonic development of Trichoplax, however, genes thought to be specific for mesoderm in bilaterian animals turned out to be already present in non-bilaterians. Searching for a Brachyury homologue, two members of the T-box gene family were isolated from Trichoplax, Brachyury and a Tbx2/3 homologue. The T-box genes encode a transcription factor family characterized by the DNA-binding T-box domain. T-box genes have been found in all metazoans so far investigated, but in contrast to other transcription factors such as the homeobox family, T-box genes are not present in plants or fungi. The distinct expression patterns of two T-box genes in Trichoplax point to non-redundant functions already present at the beginning of animal evolution. Since the expression patterns derived by in situ hybridization do not overlap with anatomical structures, it can be concluded that this simple animal has more than the four cell types described in the literature. This hidden complexity and the unresolved position in relation to Porifera, Cnidaria, Ctenophora and Bilateria highlight the necessity of the inclusion of Trichoplax in studies of comparative evolutionary and developmental biology.



Similar content being viewed by others
References
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Amacher SL, Draper BW, Summers BR, Kimmel CB (2002) The zebrafish T-box genes no tail and spadetail are required for development of trunk and tail mesoderm and medial floor plate. Development 129:3311–3323
Arendt D, Technau U, Wittbrodt J (2001) Evolution of the bilaterian larval foregut. Nature 409:81–85
Bridge D, Cunningham CW, DeSalle R, Buss LW (1995) Class-level relationships in the phylum Cnidaria: molecular and morphological evidence. Mol Biol Evol 12:679–689
Christen R, Ratto A, Baroin A, Perasso R, Grell KG, Adoutte A (1991) An analysis of the origin of metazoans, using comparisons of partial sequences of the 28S RNA, reveals an early emergence of triploblasts. EMBO J 10:499–503
Coll M, Seidman JG, Müller CW (2002) Structure of the DNA-bound T-box domain of human TBX3, a transcription factor responsible for ulnar-mammary syndrome. Structure 10:343–356
Collins AG (1998) Evaluating multiple alternative hypotheses for the origin of Bilateria: an analysis of 18S rRNA molecular evidence. Proc Natl Acad Sci USA 95:15458–15463
Collins AG (2002) Phylogeny of Medusozoa and the evolution of cnidarian life cycles. J Evol Biol 15:418–432
Ender A, Schierwater B (2003) Placozoa are not derived cnidarians: evidence from molecular morphology. Mol Biol Evol 20:130–134
Grell KG (1971) Embryonalenwicklung bei Trichoplax adhaerens F. E. Schulze. Naturwissenschaften 58:570
Grell KG, Benwitz G (1971) Die ultrastruktur von Trichoplax adhaerens F. E. Schulze. Cytobiologie 4:216–240
Grell KG, Ruthmann A (1991) Placozoa. Microscopic anatomy of invertebrates, vol 2. Wiley-Liss, New York, pp 13–27
Gröger H, Callaerts P, Gehring WJ, Schmid V (2000) Characterization and expression analysis of an ancestor-type Pax gene in the hydrozoan jellyfish Podocoryne carnea. Mech Dev 94:157–169
Holland PWH (1999) The future of evolutionary developmental biology. Nature 402 (Suppl):C41–C44
Hyman LH (1940) The invertebrates: Protozoa through Ctenophora. McGraw-Hill, New York, pp 243–245
Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ (1998) Multiple sequence alignment with Clustal X. Trends Biochem Sci 23:403–405
Kispert A, Koschorz B, Herrmann BG (1995) The T protein encoded by Brachyury is a tissue-specific transcription factor. EMBO J 14:4763–4772
Krumbach T (1907) Trichoplax, die umgewandelte Planula einer Hydromeduse. Zool Anz 31:450–454
Lartillot N, Lespinet O, Vervoort M, Adoutte A (2002) Expression pattern of Brachyury in the mollusc Patella vulgata suggests a conserved role in the establishment of the AP axis in Bilateria. Development 129:1411–1421
Miller RL (1971) Trichoplax adhaerens Schulze 1883: return of an enigma. Biol Bull 141:374
Minot CS (1883) An apparently new animal type. Science 1:305
Müller CW, Herrmann BG (1997) Crystallographic structure of the T domain-DNA complex of the Brachyury transcription factor. Nature 389:884–888
Odorico DM, Miller DJ (1997) Internal and external relationships of the Cnidaria: implications of primary and predicted secondary structure of the 5′-end of the 23S-like rDNA. Proc R Soc Lond B Biol Sci 264:77–82
Papaioannou VE (2001) T-box genes in development: from hydra to humans. Int Rev Cytol 207:1–70
Paxton C, Zhao H, Chin Y, Langner K, Reecy J (2002) Murine Tbx2 contains domains that activate and repress gene transcription. Gene 283:117–224
Philippe H, Chenuil A, Adoutte A (1994) Can the cambrian explosion be inferred through molecular phylogeny? Development 120 (Suppl):15–26
Ruppert EE, Barnes RD (1994) Invertebrate zoology, 6th edn. Saunders College Publishing, Orlando, Fla.
Ruthmann A, Wenderoth H (1975) Der DNA-gehalt der Zellen bei dem primitiven Metazoon Trichoplax adhaerens F. E. Schulze. Cytobiologie 10:421–431
Ruthmann A, Behrendt G, Wahl R (1986) The ventral epithelium of Trichoplax adhaerens (Placozoa): cytoskeletal structures, cell contacts and endocytosis. Zoomorphology 106:115–122
Ruvinsky I, Silver LM, Gibson-Brown JJ (2000) Phylogenetic analysis of T-Box genes demonstrates the importance of amphioxus for understanding evolution of the vertebrate genome. Genetics 156:1249–1257
Schierwater B, Kuhn K (1998) Homology of Hox genes and the zootype concept in early metazoan evolution. Mol Phylogenet Evol 9:375–381
Schmidt HA, Strimmer K, Vingron M, von Haeseler A (2002) TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 18:502–504
Scholz CB, Technau U (2003) The ancestral role of Brachyury: expression of NemBra1 in the basal cnidarian Nematostella vectensis (Anthozoa). Dev Genes Evol 212:563–570
Schubotz H (1912) Ist Trichoplax die umgewandelte Planula einer Hydromeduse? Zool Anz 39:582–585
Schuchert P (1993) Trichoplax adhaerens (Phylum Placozoa) has cells that react with antibodies against the neuropeptide RFamide. Acta Zool (Stockholm) 74:115–117
Schulze FE (1883) Trichoplax adhaerens, nov. gen., nov. spec. Zool Anz 6:92–97
Spring J, Yanze N, Jösch C, Middel AM, Winninger B, Schmid V (2002) Conservation of Brachyury, Mef2, and Snail in the myogenic lineage of jellyfish: a connection to the mesoderm of bilateria. Dev Biol 244:372–384
Syed T, Schierwater B (2002) The evolution of the Placozoa: a new morphological model. Senckenbergiana Lethaea 82:315–324
Takada N, Goto T, Satoh N (2002) Expression pattern of the Brachyury gene in the arrow worm Paraspadella gotoi (Chaetognatha). Genesis 32:240–245
Technau U (2001) Brachyury, the blastopore and the evolution of the mesoderm. BioEssays 23:788–794
Technau U, Bode HR (1999) HyBra1, a Brachyury homologue, acts during head formation in Hydra. Development 126:999–1010
Thiemann M, Ruthmann A (1989) Microfilaments and microtubules in isolated fiber cells of Trichoplax adhaerens (Placozoa). Zoomorphology 109:89–96
Thiemann M, Ruthmann A (1991) Alternative modes of asexual reproduction in Trichoplax adhaerens (Placozoa). Zoomorphology 110:165–174
Wainright PO, Hinkle G, Sogin ML, Stickel SK (1993) Monophyletic origins of the metazoa: an evolutionary link with fungi. Science 260:340–342
Woollard A, Hodgkin J (2000) The Caenorhabditis elegans fate-determining gene mab-9 encodes a T-box protein required to pattern the posterior hindgut. Genes Dev 14:596–603
Yanze N, Spring J, Schmidli C, Schmid V (2001) Conservation of Hox/ParaHox-related genes in the early development of a cnidarian. Dev Biol 236:89–98
Acknowledgements
We would like to thank Volker Schmid and the members of his laboratory for their help and the Swiss National Science Foundation and the Treubel-Fonds for their financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by D. Tautz
Rights and permissions
About this article
Cite this article
Martinelli, C., Spring, J. Distinct expression patterns of the two T-box homologues Brachyury and Tbx2/3 in the placozoan Trichoplax adhaerens . Dev Genes Evol 213, 492–499 (2003). https://doi.org/10.1007/s00427-003-0353-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-003-0353-5