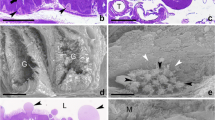
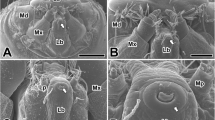
Abstract
Silk glands of the mulberry silkworm Bombyx mori are long and paired structures originating from the labial region and are anatomically and physiologically divided into three major compartments, the anterior, middle and posterior silk glands. The silk gland morphogenesis is complete by 8 days post egg laying. Extensive growth of silk glands during the larval stages is due to increase in tissue mass and not cell number. The cells in a completely formed silk gland pursue an endoreplicative cell cycle, and the genome undergoes multiple rounds of replication without mitosis or nuclear division. The expression patterns of cyclin B (mitotic cyclin) and cyclin E (G1 cyclin, essential for G1/S transition in both mitotic and endoreplicative cell cycles) in the course of silk gland development revealed that mitotic cell divisions take place only in the apex of the growing silk gland. However, the persistence of another mitotic focus in the middle silk gland even when the growing apex has moved well past this zone suggested the continued operation of mitosis for a while in this restricted region. The lack of cyclin B expression and abundance of cyclin E in the rest of the areas confirmed an alternation of the G1 and S phases of the cell cycle without an intervening mitotic phase. No expression of cyclin B was noticed anywhere in the silk glands after stage 25 of embryogenesis, indicating a complete switch over to the endomitotic mode of the cell cycle. The onset of expression of various genes encoding different silk proteins correlated with the onset of endomitotic events.






Similar content being viewed by others
References
Amanai K, Suzuki Y, Ohtaki T (1994) Involvement of maternally transcribed lectin gene in the early development of Bombyx mori. Roux's Arch Dev Biol 203:397–401
Amon A, Irniger S, Nasmyth K (1994) Closing the cell cycle in yeast: G2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell 77:1037–1050
Chevillard M, Couble P, Prudhomme J-C (1986) Complete nucleotide sequence of the gene encoding the Bombyx mori silk protein P25 and predicted amino acid sequence of the protein. Nucleic Acids Res 14:6341–6342
Couble P, Michaille J-J, Garel A, Couble M-L Prudhomme J-C (1987) Developmental switches of sericin mRNA splicing in individual cells of Bombyx mori silkglands. Dev Biol 124:431–440
Dhawan S, Gopinathan KP (2002) Molecular cloning and expression pattern of a Cubitus interruptus homologue from the mulberry silkworm Bombyx mori. Mech Dev 118:203–207
Dulic V, Lees E, Reed SI (1992) Association of human cyclin E with a periodic G1-S phase protein kinase. Science 257:1958–1961
Duronio RJ, O' Farrell PH (1995) Developmental control of the G1 to S transition in Drosophila: Cyclin E is a limiting downstream target of E2F. Genes Dev 9:1456–1468
Duronio RJ, O'Farrell PH, Xie JE, Brook A, Dyson N (1995) The transcription factor E2F is required for S phase during Drosophila embryogenesis. Genes Dev 15:1445–1455
Edgar BA, Orr-Weaver TL (2001) Endoreplication cell cycles: more for less. Cell 105:297–306
Enoch T, Carr AM, Nurse P (1992) Fission yeast genes involved in coupling mitosis to completion of DNA replication. Genes Dev 6:2035–2046
Evans T, Rosenthal ET, Youngblom J, Distel D, Hunt T (1983) Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed with each cleavage division. Cell 33:389–397
Fang F, Newport JW (1993) Distinct roles of cdk2 and cdc2 in RP-A phosphorylation during the cell cycle. J Cell Sci 106:983–994
Follette PJ, Duronio RJ, O'Farrell PH (1998) Fluctuations in Cyclin E levels are required for multiple rounds of endocycle S phase in Drosophila. Curr Biol 8:235–238
Gage LP (1974) Polyploidization of the silk gland of Bombyx mori. J Mol Biol 86:97–108
Glotzer M, Murray AW, Kirschner MW (1991) Cyclin is degraded by the ubiquitin pathway. Nature 349:132–138
Holloway SL, Glotzer M, King RW, Murray AW (1993) Anaphase is initiated by proteolysis rather than by the inactivation of maturation promoting factor. Cell 73:1393–1402
Kelly TJ, Martin GS, Forsburg SL, Stephen RJ, Russo A, Nurse P (1993) The fission yeast cdc18+ gene product couples S phase to start and mitosis. Cell 74:371–382
Knoblich J, Lehner CF (1993) Synergistic action of Drosophila cyclins A and B during the G2-M transition. EMBO J 12:65–74
Knoblich J, Sauer K, Jones L, Richardson H, Saint R, Lehner CF (1994) Cyclin E controls progression through S phase and its down regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell 77:107–120
Koff A, Giordano A, Desai D, Yamashita K, Harper JW, Elledge S, Nishimoto T, Morgan DO, Franza BR, Roberts JM (1992) Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science 257:1689–1694
Lehner CF, O'Farrell PH (1989) Expression and function of Drosophila cyclin A during embryonic cell cycle progression. Cell 56:957–968
Lehner CF, O'Farrell PH (1990) The roles of Drosophila cyclin A and cyclin B in mitotic control. Cell 61:535–547
Lilly MA, Spradling AC (1996) The Drosophila endocycle is controlled by Cyclin E and lacks a checkpoint ensuring S-phase completion. Genes Dev 10:2514–2526
Ludde T, Kubicka S, Plumpe J, Liedtke C, Manns MP, Trautwein C (2001) Ras adenovirus modulate Cyclin E protein expression and DNA synthesis after partial hepatectomy. Oncogene 30:5264–5278
MacAuley A, Cross JC, Werb Z (1998) Reprogramming the cell cycle for endoreduplication in rodent trophoblast cells. Mol Biol Cell 9:795–807
Matsunami K, Kokubo H, Ohno K, Xu P, Ueno K, Suzuki Y (1999) Embryonic silk gland development in Bombyx: molecular cloning and expression of the Bombyx trachealess gene. Dev Genes Evol 209:507–514
Michaille J-J, Garel A, Prudhomme J-C (1990) Cloning and characterization of the highly polmorphic Ser2 gene of Bombyx mori. Gene 86:177–184
Moore JD, Yang J, Truant R, Kornbluth S (1999) Nuclear import of Cdk/Cyclin complexes: identification of distinct mechanisms for import of Cdk2/Cyclin E and Cdc2/Cyclin B1. J Cell Biol 144:213–224
Niranjanakumari S, Gopinathan KP (1991) Characterisation of the DNA-polymerase-α-primase complex from the silk glands of Bombyx mori. Eur J Biochem 201:431–438
Niranjanakumari S, Gopinathan KP (1992) DNA polymerase-δ from the silkglands of Bombyx mori. J Biol Chem 267:17531–17539
Niranjanakumari S, Gopinathan KP (1993) Isolation and characterisation of DNA polymerase ε from the silk glands of Bombyx mori. J Biol Chem 268:15557–15564
Ohtsubo M, Theodoras AM, Schumacher J, Roberts JM, Pagano M (1995) Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol 15:2612–2624
Patel CV, Gopinathan KP (1991) Development stage-specific expression of fibroin in the silk worm Bombyx mori is regulated translationally. Indian J Biochem Biophys 28:521–530
Pedrix-Gillot S (1979) DNA synthesis and endomitosis in the giant nuclei of the silkgland of Bombyx mori. Biochimie 61:171–204
Pines J, Hunter T (1991a) Cyclin-dependent kinases. A new cell cycle motif? Trends Cell Biol 1:117–121
Pines J, Hunter T (1991b) Human Cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J Cell Biol 115:1–17
Reed SI (1992) The role of p34 kinases in the G1 to S phase transition. Annu Rev Cell Biol 8:529–561
Richardson HE, O'Keefe LV, Marty T, Saint R (1995) Ectopic Cyclin E expression induces premature entry into S phase and disrupts formation of in the Drosophila eye imaginal disc. Development 121:3371–3379
Saka Y, Yanagida M (1993) Fission yeast cut5+, required for S phase onset and M phase restraint, is identical to the radiation damage repair gene rad4+. Cell 74:383–393
Sauer K, Lehner CF (1995) The role of cyclin E in the regulation of entry into S-phase. Prog Cell Cycle Res 1:125–139
Sauer K, Knoblich JA, Richardson H, Lehner CF (1995) Distinct modes of cyclin E/cdc2 kinase regulation and S-phase control in mitotic and endoreduplication cycles of Drosophila embryogenesis. Genes Dev 9:1327–1339
Schnittger A, Shnobinger U, Stierhof Y, Hulskamp M (2002) Ectopic B-type cyclin expression induces mitotic cycles in endoreduplicating Arabidopsis trichomes. Cell 12:415–420
Sherr C (1994) G1 phase progression: cycling on cue. Cell 79:551–555
Singh A, Gopinathan KP (1997) Analysis of gene expression during embryonic development in mulberry silkworm Bombyx mori. Curr Sci 72:214–218
Stern B, Ried G, Clegg NJ, Grigliatti TA, Lehner CF (1993) Genetic analysis of the Drosophila cdc2 homolog. Development 117:219–232
Su TT, Follette P J, O' Farrell PH (1995) Qualifying for the license to replicate. Cell 81:825–828
Sudhakar B, Gopinathan KP (2000) Expression of cyclin E in endomitotic silk-gland cells from mulberry silkworm. Gene 257:77–85
Surana U, Amon A, Dowzer C, McGrew J, Byers B, Nasmyth K (1993) Destruction of the CDC28/CLB mitotic kinase is not required for the metaphase to anaphase transition in budding yeast. EMBO J 12:1969–1978
Takami T, Kitazawa T (1960) Normal stages of embryonic development in the silkworm Bombyx mori. Tech Bull Sericult Exp Stn 75:1-31
Weinert TA, Hartwell LH (1988) The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science 241:317–322
Weinert TA, Kiser GL, Hartwell LH (1994) Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes Dev 8:652–665
Weiss A, Herzig A, Jacobs H, Lehner CF (1998) Continuous Cyclin E expression inhibits progression through endoreduplication cycles in Drosophila. Curr Biol 8:239–242
Wuarin J, Buck V, Nurse P, Millar JB (2002) Stable association of mitotic cyclin B/Cdc2 to replication origins prevents endoreduplication. Cell 111:419–431
Acknowledgements
We thank Prof. Jean-Claude Prudhomme and Dr. Pierre Couble, University of Lyon, France for many helpful discussions, Dr. Bayar Thimmapaya, Northwestern University, Chicago, USA for anti-human Cyclin E and anti-human Actin antibodies and Dr. S. Sreekumar, Central Silk Research and Training Institute (CSB), Mysore, for the supply of silkworm embryos and larvae. S D is a recipient of a Senior Research Fellowship from CSIR, New Delhi. We thank the Department of Biotechnology, Government of India, and the Indo French Centre for Promotion of Advanced Research (IFCPAR) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by D. Tautz
Rights and permissions
About this article
Cite this article
Dhawan, S., Gopinathan, K.P. Cell cycle events during the development of the silk glands in the mulberry silkworm Bombyx mori . Dev Genes Evol 213, 435–444 (2003). https://doi.org/10.1007/s00427-003-0343-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-003-0343-7