Abstract
Main conclusion
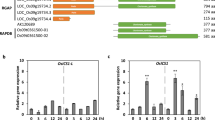
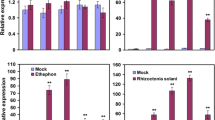
The rice protein OsWRKY6 directly activates OsWRKY45 and OsWRKY47 expression, and also activates OsPR1a and OsPR1b through the two OsWRKYs, and this transcriptional module participates in Xa1-mediated defense against the pathogen Xanthomonas oryzae pv. oryzae.
Abstract
Biotic stress, the pathogen Xanthomonas oryzae pv. oryzae (Xoo) in particular, negatively impacts worldwide productivity and yield in the staple crop rice (Oryza sativa). OsWRKY transcription factors are involved in various biotic stress responses in rice, and OsWRKY6 specifically acts as an important defense regulator against Xoo. However, the relationship between OsWRKY6 and other OsWRKYs, as well as its role in resistance (R) gene-mediated defense, have yet to be studied in depth. Here, we characterized a transcriptional cascade triggered by OsWRKY6 that regulated defense against Xoo infection mediated by the NBS-LRR protein Xa1. OsWRKY45 and OsWRKY47 were identified as direct transcriptional targets of OsWRKY6, and their two gene products reciprocally activated their two genes. Furthermore, OsWRKY6 activated OsPR1a and OsPR1b via the OsWRKY45 and OsWRKY47. Two OsWRKY6 RNAi knockdown lines showed significantly reduced defense even against an incompatible Xoo infection, and the expression of OsWRKY6 was not regulated by OsWRKY51 and OsWRKY88. This study reveals that a novel downstream transcriptional pathway activated by OsWRKY6 is involved in Xa1-mediated defense against Xoo.







Similar content being viewed by others
Abbreviations
- BLB:
-
Bacterial leaf blight
- GFP:
-
Green fluorescent protein
- rLUC:
-
Renilla luciferase
- RMP:
-
Rice mesocotyl protoplast
- Xoo :
-
Xanthomonas oryzae Pv. oryzae
References
Cheng H, Liu H, Deng Y, Xiao J, Li X, Wang S (2015) The WRKY45-2 WRKY13 WRKY42 transcriptional regulatory cascade is required for rice resistance to fungal pathogen. Plant Physiol 167(3):1087–1099
Choi C, Hwang SH, Fang IR, Kwon SI, Park SR, Ahn I, Kim JB, Hwang DJ (2015) Molecular characterization of Oryza sativa WRKY6, which binds to W-box-like element 1 of the Oryza sativa pathogenesis-related (PR) 10a promoter and confers reduced susceptibility to pathogens. New Phytol 208(3):846–859. https://doi.org/10.1111/nph.13516
Choi NY, Lee E, Lee SG, Choi CH, Park SR, Ahn I, Bae SC, Hwang CH, Hwang DJ (2017) Genome-wide expression profiling of OsWRKY superfamily genes during infection with Xanthomonas oryzae pv. oryzae using real-time PCR. Front Plant Sci 8:1628. https://doi.org/10.3389/fpls.2017.01628
Choi N, Im JH, Lee E, Lee J, Choi C, Park SR, Hwang D-J (2020) OsWRKY10 transcriptional regulatory cascades in rice are involved in basal defense and Xa1-mediated resistance. J Exp Bot 71:3735–3748
Cook AA, Walker JC, Larson RH (1952) Studies on the disease cycle of black rot of Crucifers. Phytopathology 42(3):162–167
Dai X, Wang Y, Zhang W-H (2016) OsWRKY74, a WRKY transcription factor, modulates tolerance to phosphate starvation in rice. J Exp Bot 67(3):947–960
Eulgem T, Rushton PJ, Schmelzer E, Hahlbrock K, Somssich IE (1999) Early nuclear events in plant defence signalling: rapid gene activation by WRKY transcription factors. EMBO J 18(17):4689–4699
Fukushima S, Mori M, Sugano S, Takatsuji H (2016) Transcription factor WRKY62 plays a role in pathogen defense and hypoxia-responsive gene expression in rice. Plant Cell Physiol 57(12):2541–2551
Goto S, Sasakura-Shimoda F, Suetsugu M, Selvaraj MG, Hayashi N, Yamazaki M, Ishitani M, Shimono M, Sugano S, Matsushita A, Tanabata T, Takatsuji H (2015) Development of disease-resistant rice by optimized expression of WRKY45. Plant Biotechnol J 13(6):753–765. https://doi.org/10.1111/pbi.12303
Hwang S-H, Lee IA, Yie SW, Hwang D-J (2008) Identification of an OsPR10a promoter region responsive to salicylic acid. Planta 227(5):1141–1150
Hwang S-H, Yie SW, Hwang D-J (2011) Heterologous expression of OsWRKY6 gene in Arabidopsis activates the expression of defense related genes and enhances resistance to pathogens. Plant Sci 181(3):316–323
Hwang S-H, Kwon SI, Jang J-Y, Fang IL, Lee H, Choi C, Park S, Ahn I, Bae S-c, Hwang D-J (2016) OsWRKY51, a rice transcription factor, functions as a positive regulator in defense response against Xanthomonas oryzae pv. oryzae. Plant Cell Rep 35(9):1975–1985
Im JH, Cho YH, Kim GD, Kang GH, Hong JW, Yoo SD (2014) Inverse modulation of the energy sensor Snf1-related protein kinase 1 on hypoxia adaptation and salt stress tolerance in Arabidopsis thaliana. Plant Cell Environ 37(10):2303–2312
Jang J-Y, Choi CH, Hwang D-J (2010) The WRKY superfamily of rice transcription factors. Plant Pathol J 26(2):110–114
Jiang CJ, Shimono M, Sugano S, Kojima M, Liu XQ, Inoue H, Sakakibara H, Takatsuji H (2013) Cytokinins act synergistically with salicylic acid to activate defense gene expression in rice. Mol Plant Microbe in 26(3):287–296
Koo SC, Moon BC, Kim JK, Kim CY, Sung SJ, Kim MC, Cho MJ, Cheong YH (2009) OsBWMK1 mediates SA-dependent defense responses by activating the transcription factor OsWRKY33. Biochem Biophys Res Commun 387(2):365–370
Lee H, Cha J, Choi C, Choi N, Ji HS, Park SR, Lee S, Hwang DJ (2018) Rice WRKY11 plays a role in pathogen defense and drought tolerance. Rice 11:5
Liang X, Chen X, Li C, Fan J, Guo Z (2017) Metabolic and transcriptional alternations for defense by interfering OsWRKY62 and OsWRKY76 transcriptions in rice. Sci Rep 7(1):2474
Liu XQ, Bai XQ, Wang XJ, Chu CC (2007) OsWRKY71, a rice transcription factor, is involved in rice defense response. J Plant Physiol 164(8):969–979. https://doi.org/10.1016/j.jplph.2006.07.006
Liu J, Chen X, Liang X, Zhou X, Yang F, Liu J, He SY, Guo Z (2016) Alternative splicing of rice WRKY62 and WRKY76 transcription factor genes in pathogen defense. Plant Physiol 171(2):1427–1442
Peng Y, Bartley LE, Chen X, Dardick C, Chern M, Ruan R, Canlas PE, Ronald PC (2008) OsWRKY62 is a negative regulator of basal and Xa21-mediated defense against Xanthomonas oryzae pv. oryzae in rice. Mol Plant 1(3):446–458. https://doi.org/10.1093/mp/ssn024
Peng Y, Bartley LE, Canlas P, Ronald PC (2010) OsWRKY IIa transcription factors modulate rice innate immunity. Rice 3(1):36–42. https://doi.org/10.1007/s12284-010-9039-6
Qiu D, Xiao J, Ding X, Xiong M, Cai M, Cao Y, Li X, Xu C, Wang S (2007) OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol Plant Microbe Ineract 20(5):492–499. https://doi.org/10.1094/MPMI-20-5-0492
Rice WRKY Working Group (2012) Nomenclature report on rice WRKY’s—conflict regarding gene names and its solution. Rice 5(1):3–5
Shimono M, Koga H, Akagi A, Hayashi N, Goto S, Sawada M, Kurihara T, Matsushita A, Sugano S, Jiang CJ, Kaku H, Inoue H, Takatsuji H (2012) Rice WRKY45 plays important roles in fungal and bacterial disease resistance. Mol Plant Pathol 13(1):83–94. https://doi.org/10.1111/j.1364-3703.2011.00732.x
Son S, An HK, Seol YJ, Park SR, Im JH (2020) Rice transcription factor WRKY114 directly regulates the expression of OsPR1a and Chitinase to enhance resistance against Xanthomonas oryzae pv. oryzae. Biochem Biophys Res Commun 533(4):1262–1268. https://doi.org/10.1016/j.bbrc.2020.09.141
Song Y, Jing S, Yu D (2009) Overexpression of the stress-induced OsWRKY08 improves osmotic stress tolerance in Arabidopsis. Chin Sci Bull 54(24):4671–4678
Tian X, Li X, Zhou W, Ren Y, Wang Z, Liu Z, Tang J, Tong H, Fang J, Bu Q (2017) Transcription factor OsWRKY53 positively regulates brassinosteroid signaling and plant architecture. Plant Physiol 175(3):1337–1349
Yokotani N, Sato Y, Tanabe S, Chujo T, Shimizu T, Okada K, Yamane H, Shimono M, Sugano S, Takatsuji H, Kaku H, Minami E, Nishizawa Y (2013) WRKY76 is a rice transcriptional repressor playing opposite roles in blast disease resistance and cold stress tolerance. J Exp Bot 64(16):5085–5097. https://doi.org/10.1093/jxb/ert298
Acknowledgements
This study was carried out with the support of “Research Program for Agricultural Science & Technology Development (Project No. PJ01246303)”, National Institute of Agricultural Sciences, Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
425_2022_3830_MOESM1_ESM.pptx
Supplementary file1 Fig. S1 The expression analysis of defense related genes in OsWRKY6OX and OsWRKY6KD. a Gene expression of 11 defense related OsWRKYs in OsWRKY6OX. b Gene expression of OsWRKY11, 24, 62, 77 and 104 in two OsWRKY6KD lines. Total RNA was isolated from three-week-old WT and OsWRKY6OX or OsWRKY6KD seedlings and cDNA synthesis was carried out. qPCR was performed with gene-specific primers. OsActin was used as a gene expression control. Fig. S2 Distribution of W-box and W-box-like element 1 (WLE1) motifs in OsWRKY45, 47 and 76 promoters. a OsWRKY45 promoter. b OsWRKY47 promoter. c OsWRKY76 promoter. Closed triangle: W-box; open triangle: WLE1. The letters a, b, c, and d marked on each promoter indicate the regions amplified by ChIP-qPCR. Anti-hemagglutinin (HA) antibody was used as a negative control. Fig. S3 Gene expressions of defense marker genes in OsWRKY6 and 45 and 47 overexpressing lines. Gene expression of OsPR1a, OsPR1b, BETV1 and Chitianse were analyzed in OsWRKY6OX (a), OsWRKY45OX (b) and OsWRKY47OX (c). Total RNA was isolated from the WT and designated transgenics and cDNA was synthesized with it. The gene expressions were determined with qPCR with gene-specific primers. OsActin was used as an expression control. Values are means of triplicate-independent biological repeats with SEs. ** P < 0.001, * P < 0.01, Fig. S4 Chromatin immunoprecipitation (ChIP)-qPCR of OsPR1a and OsPR1b promoters by OsWRKY6. a and b The schematic draw of OsPR1a (a) and OsPR1b (b) promoters. Closed triangle: W-box; open triangle: WLE1. The letters a and b marked on each promoter indicate the regions amplified by ChIP-qPCR. c ChIP-qPCR result of OsPR1a. d ChIP-qPCR result of OsPR1b. Anti-hemagglutinin (HA) antibody was used as a negative control. Fig. S5 Gene expression analysis of OsWRKY6 in OsWRKY45OX and OsWRKY47OX. Total RNA was isolated from three-week-old WT and two transgenic lines of OsWRKY45 and OsWRKY47. cDNA was synthesized with the RNA and OsWRKY6 expressions were determined with specific primers. OsActin was used as an expression control. Table S1 List of primers in this study (PPTX 173 KB)
Rights and permissions
About this article
Cite this article
Im, J.H., Choi, C., Park, S.R. et al. The OsWRKY6 transcriptional cascade functions in basal defense and Xa1-mediated defense of rice against Xanthomonas oryzae pv. oryzae. Planta 255, 47 (2022). https://doi.org/10.1007/s00425-022-03830-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-022-03830-5