Abstract
Main conclusion
In this study, useful hybrid promoters were developed for efficient ectopic gene expression in monocot and dicot plants, and they hold strong prominence in both transgenic research and biotech industries.
Abstract
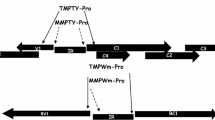
This study deals with developing novel synthetic promoters derived from Rice Tungro Bacilliform Virus (RTBV) and Mirabilis Mosaic Virus (MMV). Despite numerous availability, there is a severe scarcity of promoters universally suitable for monocot and dicot plants. Here, eight chimeric promoter constructs were synthesized as gBlocks gene fragments through domain swapping and hybridization by incorporating important domains of previously characterized RTBV and MMV promoters. The developed promoter constructs were assessed for transient GUS expression in tobacco protoplast (Xanthi Brad) and agro-infiltrated tobacco, petunia, rice and pearl millet. Protoplast expression analysis showed that two promoter constructs, namely pUPMA-RP1-MP1GUS and pUPMA-RP4-MP1GUS exhibited 3.56 and 2.5 times higher activities than that of the CaMV35S promoter. We had observed the similar type of expression patterns of these promoters in agroinfiltration-based transient studies. RP1-MP1 and RP4-MP1 promoters exhibited 1.87- and 1.68-fold increase expression in transgenic tobacco plants; while, a 1.95-fold increase was found in RP1-MP1 transgenic rice plants when compared their activities with CaMV35S promoter. Furthermore, on evaluating these promoter constructs for their expression in the bacterial system, pUPMA-RP1-MP1GFP was found to have the highest GFP expression. Moreover, the promoter construct was also evaluated for its capacity to express the HMP3 gene. Biobeads of encapsulated bacterial cells expressing HMP3 gene under control of the pUPMA-RP4-MP1 promoter were found to reduce 72.9% copper and 29.2% zinc concentration from wastewater. Our results had demonstrated that the developed promoter constructs could be used for translational research in dicot, monocot plants and bacterial systems for efficient gene expression.








Similar content being viewed by others
References
Acharya S, Sengupta S, Patro S, Purohit SK, Maiti IB, Dey N (2014a) Development of an intra-molecularly shuffled efficient chimeric plant promoter from plant infecting Mirabilis mosaic virus promoter sequence. J Biotechnol 169:103–111
Acharya S, Ranjan R, Pattanaik S, Samal SK, Maiti IB, Dey N (2014b) Efficient chimeric plant promoters derived from plant infecting viral promoter sequences. Planta 239:381–396
Ali S, Kim WC (2019) A fruitful decade using synthetic promoters in the improvement of transgenic plants. Front Plant Sci 10:1433. https://doi.org/10.3389/fpls.2019.01433
Andrieu A, Breitler JC, Sire C, Meynard D, Gantet P, Guiderdoni E (2012) An in planta, Agrobacterium-mediated transient gene expression method for inducing gene silencing in rice (Oryza sativa L.) leaves. Rice (N Y) 5:23. https://doi.org/10.1186/1939-8433-5-23
Azuma M, Morimoto R, Hirose M, Morita Y, Hoshino A, Iida S (2016) A petal-specific InMYB1 promoter from Japanese morning glory: a useful tool for molecular breeding of floricultural crops. Plant Biotechnol J 14:354–363
Bhullar S, Chakravarthy S, Advani S, Datta S, Pental D, Burma PK (2003) Strategies for development of functionally equivalent promoters with minimum sequence homology for transgene expression in plants: cis-elements in a novel DNA context versus domain swapping. Plant physiol 132:988–998
Bhullar S, Datta S, Advani S, Chakravarthy S, Gautam T, Pental D, Burma PK (2007) Functional analysis of cauliflower mosaic virus 35S promoter: re-evaluation of the role of subdomains B5, B4 and B2 in promoter activity. Plant Biotechnol J 5:696–708
Cai Y, Kallam K, Tidd H, Gendarini G, Salzman A, Patron NJ (2020) Rational design of minimal synthetic promoters for plants. bioRxiv. https://doi.org/10.1101/2020.05.14.095406
Chen H, Nelson RS, Sherwood JL (1994) Enhanced recovery of transformants of Agrobacterium tumefaciens after freeze-thaw transformation and drug selection. Biotechniques 16:664–668
de Mesa MC, Santiago-Doménech N, Pliego-Alfaro F, QuesadaMA MercadoJA (2004) The CaMV 35S promoter is highly active on floral organs and pollen of transgenic strawberry plants. Plant Cell Report 23:32–38
Deb D, Shrestha A, Maiti IB, Dey N (2018) Recombinant promoter (MUASCsV8CP) driven totiviral killer protein 4 (KP4) imparts resistance against fungal pathogens in transgenic tobacco. Front Plant Sci 5:278. https://doi.org/10.3389/fpls.2018.00278
Dey N, Maiti IB (1999) Structure and promoter/leader deletion analysis of mirabilis mosaic virus (MMV) full-length transcript promoter in transgenic plants. Plant Mol Bio 40:771–782
Dey N, Sarkar S, Acharya S, Maiti IB (2015) Synthetic promoters in planta. Planta 242:1077–1094
Fraiture M, Zheng X, Brunner F (2014) An Arabidopsis and tomato mesophyll protoplast system for fast identification of early MAMP-triggered immunity-suppressing effectors. Methods Mol Biol 1127:213–230
Furtado A, Henry RJ, Pellegrineschi A (2009) Analysis of promoters in transgenic barley and wheat. Plant Biotechnol J 7:240–253
Gudynaite-Savitch L, Johnson DA, Miki BL (2009) Strategies to mitigate transgene–promoter interactions. Plant Biotechnol J 7:472–485
Gupta D, Ranjan R (2017) In silico comparative analysis of promoters derived from plant pararetroviruses. VirusDisease 28:416–421
Gupta D, Ranjan R (2020) In silico characterization of synthetic promoters designed from mirablilis mosaic virus and rice tungro bacilliform virus. VirusDisease 31(369):373
Gupta D, Satpati S, Dixit A, Ranjan R (2019) Fabrication of biobeads expressing heavy metal binding protein for removal of heavy metal from waste water. Appl Microbiol Biotechnol 103:5411. https://doi.org/10.1007/s00253-019-09852-6
Han YJ, Kim YM, Hwang OJ, Kim JI (2015) Characterization of a small constitutive promoter from Arabidopsis translationally controlled tumor protein (AtTCTP) gene for plant transformation. Plant Cell Rep 34:265–275
Hepler P, Brain Gunning ES (1998) Confocal fluorescenece microscopy of plant cells. Protoplasma 201:121–157
Higo K, Ugawa Y, Iwamoto M, Higo H (1998) PLACE: a database of plant cis-acting regulatory DNA elements. Nucleic Acids Res 26:358–359
Jefferson RA (2000) Assaying chimeric genes in plants:the GUS gene fusion system. Plant Mol Biol Rep 5:387–405
Khan A, Shrestha A, Bhuyan K, Maiti IB, Dey N (2018) Structural characterization of a novel full-length transcript promoter from Horseradish Latent Virus (HRLV) and its transcriptional regulation by multiple stress responsive transcription factors. Plant Mol Biol 96:179–196
Kim KN, Guiltinan MJ (1999) Identification of cis-acting elements important for expression of the starch-branching enzyme I gene in maize endosperm. Plant Physiol 121:225–236
Kohli A, Griffiths N, Palacios N, Twyman RM, Vain P (1999) Molecular characterization of transforming plasmid rearrangements in transgenic rice reveals a recombination hotspot in the CaMV 35S promoter and confirms the predominance of microhomology mediated recombination. Plant J: Cell Mol Biol 17:591–601
Kumar D, Patro S, Ghosh J, Das A, Maiti IB, Dey N (2012) Development of a salicylic acid inducible minimal sub-genomic transcript promoter from Figwort mosaic virus with enhanced root- and leaf-activity using TGACG motif rearrangement. Gene 503:36–47
Li M, Song B, Zhang Q, Liu X, Lin Y, Ou Y, Zhang H, Liu J (2013) A synthetic tuber-specific and cold-induced promoter is applicable in controlling potato cold-induced sweetening. Plant Physiol Biochem 67:41–47
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔCT method. Methods 25:402–408
Maiti IB, Murphy JF, Shaw JG, Hunt AG (1993) Plants that express a potyvirus proteinase gene are resistant to virus infection. Proc Natl Acad Sci USA 90:6110–6114
Mathur S, Dasgupta I (2007) Downstream promoter sequence of an Indian isolate of Rice tungro bacilliform virus alters tissue-specific expression in host rice and acts differentially in heterologous system. Plant Mol Biol 65:259–275
Matsuda D, Dreher TW (2004) The tRNA-like structure of Turnip yellow mosaic virus RNA is a 3′-translational enhancer. Virology 321:360–446
Mehrotra R, Kiran K, Chaturvedi CP, Ansari SA, Lodhi N, Sawant S, Tuli R (2005) Effect of copy number and spacing of the ACGT and GT cis elements on transient expression of minimal promoter in plants. J Genet 84:183–187
Mitsuhara I, Ugaki M, Hirochika H, Ohshima M, Murakami T, Gotoh Y, Katayose Y, Nakamura S, Honkura R, Nishimiya S, Ueno K, Mochizuki A, Tanimoto H, Tsugawa H, Otsuki Y, Ohashi Y (1996) Efficient promoter cassettes for enhanced expression of foreign genes in dicotyledonous and monocotyledonous plants. Plant Cell Physiol 37:49–59
Mondal T, Bhattacharya A, Ahuja P, Chand P (2001) Transgenic tea [Camellia sinensis (L.) O. Kuntze cv. Kangra Jat] plants obtained by Agrobacterium-mediated transformation of somatic embryos. Plant Cell Report 20:712–720
Nguyen VC, Nguyen VK, Singh CH, Devi GS, Reddy VS, Leelavathi S (2017) Fast recovery of transgenic submergence tolerant rice cultivars of North-East India by early co-cultivation of Agrobacterium with pre-cultured callus. Physiol Mol Biol Plants 23:115–123
Patro S, Kumar D, Ranjan R, Maiti IB, Dey N (2012) The development of efficient plant promoters for transgene expression employing plant virus promoters. Mol Plant 5:941–944
Peremarti A, Twyman RM, Galera SG, Naqvi S, Farre G, Sabalza M, Miralpeix B, Dashevskaya S, Yuan D, Ramessar K, Christou P, Zhu C, Bassie L, Capell T (2010) Promoter diversity in multigene transformation. Plant Mol Biol 73:363–378
Potenza C, Aleman L, Sengupta-Gopalan C (2004) Targeting transgene expression in research, agricultural, and environmental applications: promoters used in plant transformation. Vitro Cell Dev Biol 40:1–22
Ranjan R, Dey N (2012) Development of vascular tissue and stress inducible hybrid-synthetic promoters through dof-1 motifs rearrangement. Cell Biochem Biophys 63:235–245
Ranjan R, Patro S, Kumari S, Kumar D, Dey N, Maiti IB (2011) Efficient chimeric promoters derived from full-length and sub-genomic transcript promoters of Figwort mosaic virus (FMV). J Biotechnol 152:58–62
Rushton PJ, Reinstadler A, Lipka V, Lippok B, Somssich IE (2002) Synthetic plant promoters containing defined regulatory elements provide novel insights into pathogen- and wound-induced signaling. Plant Cell 14:749–762
Sahoo DK, Ranjan R, Kumar D, Kumar A, Sahoo BS, Raha S, Maiti IB, Dey N (2009) An alternative method of promoter assessment by confocal laser scanning microscopy. J Virol Methods 161:114–121
Shah SH, Jan SA, Ahmad N, Khan SU, Kumar T, Iqbal A, Nasir F, Noman M, Ali A (2015) Use of different promoters in transgenic plant development: current challenges and future perspectives. Am-Eurasian J Agric Environ Sci 15:664–675
Sheen J (2001) Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol 127:1466–1475
Shrestha A, Khan A, Dey N (2018) Cis-trans engineering: advances and perspectives on customized transcriptional regulation in plants. Mol Plant 11:886–898
Tao YB, He LL, Niu LJ, Xu ZF (2015) Isolation and characterization of an ubiquitin extension protein gene (JcUEP) promoter from Jatropha curcas. Planta 241:823–836
Toriumi S, Saito T, Hosokawa T, Takahashi Y, Numata T, Kurasaki M (2005) Metal binding ability of metallothionein-3 expressed in Escherichia coli. Pharmacol Toxicol 96:295–301
Vaucheret H, Fagard M (2001) Transcriptional gene silencing in plants: targets, inducers and regulators. Trends Genet 17:29–35
Wymer C, Pendle A, Boudonck K, Lloyd C (1999) Confocal microscopy of plant cells. Methods Mol Biol 122:103–130
Xie M, He Y, Gan S (2001) Bi directionalization of polar promoters in plants. Nat Biotechnol 19:677–679
Yang Y, Li R, Qi M (2000) In vivo analysis of plant promoters and transcription factors, by agroinfiltration of tobacco leaves. Plant J 22:543–551
Yang X, Boehm JS, Yang X, Salehi-Ashtiani K, Hao T, Shen Y, Lubonja R, Thomas SR, Alkan O, Bhimdi T, Green TM, Johannessen CM, Silver SJ, Nguyen C, Murray RR, Hieronymus H, Balcha D, Fan C, Lin C, Ghamsari L, Vidal M, Hahn WC, Hill DE, Root DE (2011) A public genome-scale lentiviral expression library of human ORFs. Nat Methods 8:659–661
Zhang H, Hou J, Jiang P, Qi S, Xu C, He Q (2016) Identification of a 467 bp promoter of maize phosphatidylinositol synthase gene (ZmPIS) which confers high-level gene expression and salinity or osmotic stress inducibility in transgenic tobacco. Front Plant Sci 7:42
Acknowledgments
Authors RR and DG greatly acknowledge DAE-BRNS, Government of India, Department of Atomic Energy, Mumbai, for providing financial assistance vide 37(1)/14/40/20l4-BRNS/l423 dated 24/08/20l4; Director, DEI, for providing infrastructure; and Prof. Indranil Dasgupta and Prof. R.C. Bhattacharya for providing the vector and facility for research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare no conflict of Interest.
Additional information
Communicated by Anastasios Melis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gupta, D., Dey, N., Leelavathi, S. et al. Development of efficient synthetic promoters derived from pararetrovirus suitable for translational research. Planta 253, 42 (2021). https://doi.org/10.1007/s00425-021-03565-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-021-03565-9