Abstract
Main conclusion
Whereas proline accumulates through de novo biosynthesis in plants subjected to osmotic stress, leucine, isoleucine, and valine accumulation in drought-stressed Arabidopsis thaliana is caused by abscisic acid-regulated protein degradation.
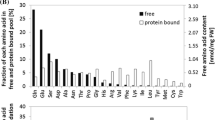
In response to several kinds of abiotic stress, plants greatly increase their accumulation of free amino acids. Although stress-induced proline increases have been studied the most extensively, the fold-increase of other amino acids, in particular branched-chain amino acids (BCAAs; leucine, isoleucine, and valine), is often higher than that of proline. In Arabidopsis thaliana (Arabidopsis), BCAAs accumulate in response to drought, salt, mannitol, polyethylene glycol, herbicide treatment, and nitrogen starvation. Plants that are deficient in abscisic acid signaling accumulate lower amounts of BCAAs, but not proline and most other amino acids. Previous bioinformatic studies had suggested that amino acid synthesis, rather than protein degradation, is responsible for the observed BCAA increase in osmotically stressed Arabidopsis. However, whereas treatment with the protease inhibitor MG132 decreased drought-induced BCAA accumulation, inhibition of BCAA biosynthesis with the acetolactate synthase inhibitors chlorsulfuron and imazapyr did not. Additionally, overexpression of BRANCHED-CHAIN AMINO ACID TRANSFERASE2 (BCAT2), which is upregulated in response to osmotic stress and functions in BCAA degradation, decreased drought-induced BCAA accumulation. Together, these results demonstrate that BCAA accumulation in osmotically stressed Arabidopsis is primarily the result of protein degradation. After relief of the osmotic stress, BCAA homeostasis is restored over time by amino acid degradation involving BCAT2. Thus, drought-induced BCAA accumulation is different from that of proline, which is accumulated due to de novo synthesis in an abscisic acid-independent manner and remains elevated for a more prolonged period of time after removal of the osmotic stress.







Similar content being viewed by others
Abbreviations
- BCAAs:
-
Branched-chain amino acids
- BCAT2:
-
BRANCHED-CHAIN AMINO ACID TRANSFERASE2
References
Angelovici R, Lipka AE, Deason N, Gonzalez-Jorge S, Lin H, Cepela J, Buell R, Gore MA, Dellapenna D (2013) Genome-wide analysis of branched-chain amino acid levels in Arabidopsis seeds. Plant Cell 25(12):4827–4843
Barnett NM, Naylor AW (1966) Amino acid and protein metabolism in Bermuda grass during water stress. Plant Physiol 41(7):1222–1230
Cao Y, Xiang X, Geng M, You Q, Huang X (2017) Effect of HbDHN1 and HbDHN2 genes on abiotic stress responses in Arabidopsis. Front Plant Sci 8:470
Cohen H, Israeli H, Matityahu I, Amir R (2014) Seed-specific expression of a feedback-insensitive form of CYSTATHIONINE-gamma-SYNTHASE in Arabidopsis stimulates metabolic and transcriptomic responses associated with desiccation stress. Plant Physiol 166(3):1575–1592
Cooke RJ, Oliver J, Davies DD (1979) Stress and protein turnover in Lemna minor. Plant Physiol 64(6):1109–1113
de Ollas C, Arbona V, Gomez-Cadenas A (2015) Jasmonoyl isoleucine accumulation is needed for abscisic acid build-up in roots of Arabidopsis under water stress conditions. Plant Cell Environ 38(10):2157–2170
Frolov A, Bilova T, Paudel G, Berger R, Balcke GU, Birkemeyer C, Wessjohann LA (2017) Early responses of mature Arabidopsis thaliana plants to reduced water potential in the agar-based polyethylene glycol infusion drought model. J Plant Physiol 208:70–83
Girousse C, Bournoville R, Bonnemain JL (1996) Water deficit-induced changes in concentrations in proline and some other amino acids in the phloem sap of alfalfa. Plant Physiol 111(1):109–113
Guo Y, Gan SS (2012) Convergence and divergence in gene expression profiles induced by leaf senescence and 27 senescence-promoting hormonal, pathological and environmental stress treatments. Plant Cell Environ 35(3):644–655
Jander G, Joshi V (2009) Aspartate-derived amino acid biosynthesis in Arabidopsis thaliana. In: Last RL (ed) The Arabidopsis book. American Society for Plant Biologists, Rockville. doi:10.1199/tab.0121
Kim DY, Scalf M, Smith LM, Vierstra RD (2013) Advanced proteomic analyses yield a deep catalog of ubiquitylation targets in Arabidopsis. Plant Cell 25(5):1523–1540
Kovacs Z, Simon-Sarkadi L, Vashegyi I, Kocsy G (2012) Different accumulation of free amino acids during short- and long-term osmotic stress in wheat. Sci World J 2012:216521
Less H, Galili G (2008) Principal transcriptional programs regulating plant amino acid metabolism in response to abiotic stresses. Plant Physiol 147(1):316–330
Martinelli T, Whittaker A, Bochicchio A, Vazzana C, Suzuki A, Masclaux-Daubresse C (2007) Amino acid pattern and glutamate metabolism during dehydration stress in the ‘resurrection’ plant Sporobolus stapfianus: a comparison between desiccation-sensitive and desiccation-tolerant leaves. J Exp Bot 58(11):3037–3046
Mishra RC, Richa, Grover A (2016) Constitutive over-expression of rice ClpD1 protein enhances tolerance to salt and desiccation stresses in transgenic Arabidopsis plants. Plant Sci 250:69–78
Murashige T, Skoog FA (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nambara E, Kawaide H, Kamiya Y, Naito S (1998) Characterization of an Arabidopsis thaliana mutant that has a defect in ABA accumulation: ABA-dependent and ABA-independent accumulation of free amino acids during dehydration. Plant Cell Physiol 39(8):853–858
Parida AK, Das AB, Mittra B, Mohanty P (2004) Salt-stress induced alterations in protein profile and protease activity in the mangrove Bruguiera parviflora. Z Naturforsch C 59(5–6):408–414
Pratelli R, Pilot G (2014) Regulation of amino acid metabolic enzymes and transporters in plants. J Exp Bot 65(19):5535–5556
Rai VK (2002) Role of amino acids in plant responses to stresses. Biol Plant 45(4):481–487
Ranieri A, Bernardi R, Lanese P, Soldatini GF (1989) Changes in free amino acid content and protein pattern of maize seedlings under water stress. Environ Exp Biol 29(3):351–357
Rhodes D, Handa S, Bressan RA (1986) Metabolic changes associated with adaptation of plant cells to water stress. Plant Physiol 82(4):890–903
Sanchez DH, Siahpoosh MR, Roessner U, Udvardi M, Kopka J (2008) Plant metabolomics reveals conserved and divergent metabolic responses to salinity. Physiol Plant 132(2):209–219
Shen L, Foster JG, Orcutt DM (1989) Composition and distribution of free amino acids in flatpea (Lathyrus sylvestris L.) as influenced by water deficit and plant age. J Exp Bot 40:71–79
Stroeher VL, Maclagan JL, Good AG (1997) Molecular cloning of a Brassica napus cysteine protease gene inducible by drought and low temperature stress. Physiol Plant 101(2):389–397
Székely G, Abrahám E, Cséplo A, Rigó G, Zsigmond L, Csiszar J, Ayaydin F, Strizhov N, Jásik J, Schmelzer E, Koncz C, Szabados L (2008) Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J 53(1):11–28
Trontin C, Kiani S, Corwin JA, Hematy K, Yansouni J, Kliebenstein DJ, Loudet O (2014) A pair of receptor-like kinases is responsible for natural variation in shoot growth response to mannitol treatment in Arabidopsis thaliana. Plant J 78(1):121–133
Urano K, Maruyama K, Ogata Y, Morishita Y, Takeda M, Sakurai N, Suzuki H, Saito K, Shibata D, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K (2009) Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J 57(6):1065–1078
Urano K, Maruyama K, Jikumaru Y, Kamiya Y, Yamaguchi-Shinozaki K, Shinozaki K (2017) Analysis of plant hormone profiles in response to moderate dehydration stress. Plant J 90(1):17–36
Verslues PE, Sharma S (2010) Proline metabolism and its implications for plant-environment interaction. Arabidopsis Book 8:e0140. doi:10.1199/tab.0140
Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J 45(4):523–539
Widodo Patterson JH, Newbigin E, Tester M, Bacic A, Roessner U (2009) Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance. J Exp Bot 60(14):4089–4103
Yan J, Lipka AE, Schmelz EA, Buckler ES, Jander G (2015) Accumulation of 5-hydroxynorvaline in maize (Zea mays) leaves is induced by insect feeding and abiotic stress. J Exp Bot 66:593–602
Acknowledgements
This work was supported by the United States National Science Foundation [Grant Number MCB-1022017].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, T., Jander, G. Abscisic acid-regulated protein degradation causes osmotic stress-induced accumulation of branched-chain amino acids in Arabidopsis thaliana . Planta 246, 737–747 (2017). https://doi.org/10.1007/s00425-017-2727-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-017-2727-3