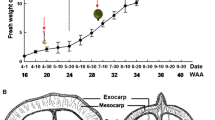
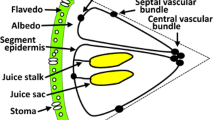
Abstract
Phloem unloading is thought to switch from a symplastic route to an apoplastic route at the beginning of ripening in grape berries and some other fleshy fruits. However, it is unclear whether different solutes accumulate in both the mesocarp vacuoles and the apoplast. We modified a method developed for tomato fruit to extract apoplastic sap from grape berries and measured the changes in apoplastic and vacuolar pH, soluble sugars, organic acids, and potassium in ripening berries of Vitis vinifera ‘Merlot’ and V. labruscana ‘Concord’. Solute accumulation varied by genotype, compartment, and chemical species. The apoplast pH was substantially higher than the vacuolar pH, especially in Merlot (approximately two units). However, the vacuole–apoplast proton gradient declined during ripening and in Merlot, but not in Concord, collapsed entirely at maturity. Hexoses accumulated in both the vacuoles and apoplast but at different rates. Organic acids, especially malate, declined much more in the vacuoles than in the apoplast. Potassium accumulated in the vacuoles and apoplast of Merlot. In Concord, by contrast, potassium increased in the vacuoles but decreased in the apoplast. These results suggest that solutes in the fruit apoplast are tightly regulated and under developmental control.






Similar content being viewed by others
Abbreviations
- D e :
-
Duration of pressurization
- P g :
-
Applied gas pressure
- fru:
-
Fructose
- glc:
-
Glucose
- suc:
-
Sucrose
- s :
-
Mesocarp solute concentration
- s a :
-
Apoplast solute concentration
- s v :
-
Vacuole solute concentration
- σ :
-
Membrane reflection coefficient
- Ψleaf :
-
Leaf xylem water potential
- V a :
-
Apoplast sap volume
References
Almeida DPF, Huber DJ (1999) Apoplastic pH and inorganic ion levels in tomato fruit: a potential means for regulation of cell wall metabolism during ripening. Physiol Plant 105:506–512
Bargel H, Neinhuis C (2005) Tomato (Lycopersicon esculentum Mill.) fruit growth and ripening as related to the biomechanical properties of fruit skin and isolated cuticle. J Exp Bot 56:1049–1060
Barnavon L, Doco T, Terrier N, Ageorges A, Romieu C, Pellerin P (2000) Analysis of cell wall neutral sugar composition, β-galactosidase activity and a related cDNA clone throughout the development of Vitis vinifera grape berries. Plant Physiol Biochem 38:289–300
Carpaneto A, Geiger D, Bamberg E, Sauer N, Fromm J, Hedrich R (2005) Phloem-localized, proton-coupled sucrose carrier ZmSUT1 mediates sucrose efflux under the control of the sucrose gradient and the proton motive force. J Biol Chem 280:21437–21443
Chen LQ, Qu XQ, Hou BH, Sosso D, Osorio S, Fernie AR, Frommer WB (2012) Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 335:207–211
DeBolt S, Hardie J, Tyerman S, Ford CM (2004) Composition and synthesis of raphide crystals and druse crystals in berries of Vitis vinifera L. cv. Cabernet Sauvignon: ascorbic acid as precursor for both oxalic and tartaric acids as revealed by radiolabelling studies. Aust J Grape Wine Res 10:134–142
Diakou P, Carde JP (2001) In situ fixation of grape berries. Protoplasma 218:225–235
Etienne A, Génard M, Lobit P, Mbeguié-A-Mbéguié D, Bugaud C (2013) What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J Exp Bot 64:1451–1469
Etxeberria E, González P, Tomlinson P, Pozueta-Romero J (2005) Existence of two parallel mechanisms for glucose uptake in heterotrophic plant cells. J Exp Bot 56:1905–1912
Fontes N, Côrte-Real M, Gerós H (2011a) New observations on the integrity, structure, and physiology of flesh cells from fully ripened grape berry. Am J Enol Vitic 62:279–284
Fontes N, Gerós H, Delrot S (2011b) Grape berry vacuole: a complex and heterogeneous membrane system specialized in the accumulation of solutes. Am J Enol Vitic 62:270–278
Fuentes S, Sullivan W, Tilbrook J, Tyerman S (2010) A novel analysis of grapevine berry tissue demonstrates a variety-dependent correlation between tissue vitality and berry shrivel. Aust J Grape Wine Res 16:327–336
Gillaspy G, Ben-David H, Gruissem W (1993) Fruits: a developmental perspective. Plant Cell 5:1439–1451
Hafke JB, van Amerongen JK, Kelling F, Furch ACU, Gaupels F, van Bel AJE (2005) Thermodynamic battle for photosynthate acquisition between sieve tubes and adjoining parenchyma in transport phloem. Plant Physiol 138:1527–1537
Hanana M, Cagnac O, Yamaguchi T, Hamdi S, Ghorbel A, Blumwald E (2007) A grape berry (Vitis vinifera L.) cation/proton antiporter is associated with berry ripening. Plant Cell Physiol 48:804–811
Hardie WJ, O’Brien TP, Jaudzems VG (1996) Morphology, anatomy and development of the pericarp after anthesis in grape, Vitis vinifera L. Aust J Grape Wine Res 2:97–142
Hawker JS, Ruffner HP, Walker RR (1976) The sucrose content of some Australian grapes. Am J Enol Vitic 27:25–129
Hayes MA, Davies C, Dry IB (2007) Isolation, functional characterization, and expression analysis of grapevine (Vitis vinifera L.) hexose transporters: differential roles in sink and source tissues. J Exp Bot 58:1985–1997
Holbrook NM, Burns MJ, Field CB (1995) Negative xylem pressures in plants: a test of the balancing pressure technique. Science 270:1193–1194
Hu L, Sun H, Li R, Zhang L, Wang S, Sui X, Zhang Z (2011) Phloem unloading follows an extensive apoplasmic pathway in cucumber (Cucumis sativus L.) fruit from anthesis to marketable maturing stage. Plant Cell Environ 34:1835–1848
Jachetta JJ, Appleby AP, Boersma L (1986) Use of the pressure vessel to measure concentrations of solutes in apoplastic and membrane-filtered symplastic sap in sunflower leaves. Plant Physiol 82:995–999
Keller M (2010) The science of grapevines—anatomy and physiology. Elsevier Academic Press, Burlington
Keller M, Smith JP, Bondada BR (2006) Ripening grape berries remain hydraulically connected to the shoot. J Exp Bot 57:2577–2587
Kramer EM (2006) How far can a molecule of weak acid travel in the apoplast or xylem? Plant Physiol 141:1233–1236
Krasnow M, Matthews M, Shackel K (2008) Evidence for substantial maintenance of membrane integrity and cell viability in normally developing grape (Vitis vinifera L.) berries throughout development. J Exp Bot 59:849–859
Lalonde S, Tegeder M, Throne-Holst M, Frommer WB, Patrick JW (2003) Phloem loading and unloading of sugars and amino acids. Plant Cell Environ 26:37–56
Lund ST, Peng FY, Nayar T, Reid KE, Schlosser J (2008) Gene expression analyses in individual grape (Vitis vinifera L.) berries during ripening initiation reveal that pigmentation intensity is a valid indicator of developmental staging within the cluster. Plant Mol Biol 68:301–315
Lüttge U, Smith JAC (1984) Mechanism of passive malic-acid efflux from vacuoles of the CAM plant Kalanchoë daigremontiana. J Membr Biol 81:149–158
Martinoia E, Maeshima M, Neuhaus HE (2007) Vacuolar transporters and their essential role in plant metabolism. J Exp Bot 58:83–102
Martinoia E, Meyer S, De Angeli A, Nagy R (2012) Vacuolar transporters in their physiological context. Annu Rev Plant Biol 63:183–213
Milner ID, Ho LC, Hall JL (1995) Properties of proton and sugar transport at the tonoplast of tomato (Lycopersicon esculentum) fruit. Physiol Plant 94:399–410
Patrick JW (1997) Phloem unloading: sieve element unloading and post-sieve element transport. Annu Rev Plant Physiol Plant Mol Biol 48:191–222
Patrick JW, Zhang W, Tyerman SD, Offler CE, Walker NA (2001) Role of membrane transport in phloem translocation of assimilates and water. Aust J Plant Physiol 28:695–707
Pilati S, Perazzolli M, Malossini A, Cestaro A, Demattè L, Fontana P, Dal Ri A, Viola R, Velasco R, Moser C (2007) Genome-wide transcriptional analysis of grapevine berry ripening reveals a set of genes similarly modulated during three seasons and the occurrence of an oxidative burst at veraison. BMC Genomics 8:428. doi:10.1186/1471-2164-8-428
Pomper KW, Breen PJ (1995) Levels of apoplastic solutes in developing strawberry fruit. J Exp Bot 46:743–752
Popp M, Lied W, Meyer AJ, Richter A, Schiller P, Schwitte H (1996) Sample preservation for determination of organic compounds: microwave versus freeze-drying. J Exp Bot 47:1469–1473
Roitsch T, González MC (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9:606–613
Ruan YL, Patrick JW (1995) The cellular pathway of postphloem sugar transport in developing tomato fruit. Planta 196:434–444
Ruan YL, Mate C, Patrick JW, Brady CJ (1995) Non-destructive collection of apoplastic fluid from developing tomato fruit using a pressure dehydration procedure. Aust J Plant Physiol 22:761–769
Ruan YL, Patrick JW, Brady CJ (1996) The composition of apoplast fluid recovered from intact developing tomato fruit. Aust J Plant Physiol 23:9–13
Sarry JE, Sommerer N, Sauvage FX, Bergoin A, Rossignol M, Albagnac G, Romieu C (2004) Grape berry biochemistry revisited upon proteomic analysis of the mesocarp. Proteomics 4:201–215
Storey R (1987) Potassium localization in the grape berry pericarp by energy-dispersive X-ray microanalysis. Am J Enol Vitic 38:301–309
Terrier N, Sauvage FX, Ageorges A, Romieu C (2001) Changes in acidity and in proton transport at the tonoplast of grape berries during development. Planta 213:20–28
Thompson MV, Holbrook NM (2004) Scaling phloem transport: information transmission. Plant Cell Environ 27:509–519
Tilbrook J, Tyerman SD (2008) Cell death in grape berries: varietal differences linked to xylem pressure and berry weight loss. Funct Plant Biol 35:173–184
Turner NC (1988) Measurement of plant water status by the pressure chamber technique. Irrig Sci 9:289–308
Turner NC, Long MJ (1980) Errors arising from rapid water loss in the measurement of leaf water potential by the pressure chamber technique. Aust J Plant Physiol 7:427–537
Tyree MT, Hammel HT (1972) The measurement of the turgor pressure and the water relations of plants by the pressure-bomb technique. J Exp Bot 23:267–282
Wada H, Shackel KA, Matthews MA (2008) Fruit ripening in Vitis vinifera: apoplastic solute accumulation accounts for pre-veraison turgor loss in berries. Planta 227:1351–1361
Wei C, Tyree MT, Bennink JP (2000) The transmission of gas pressure to xylem fluid pressure when plants are inside a pressure bomb. J Exp Bot 51:309–316
Wiedemann P, Neinhuis C (1998) Biomechanics of isolated plant cuticles. Bot Acta 111:28–34
Wu GL, Zhang XY, Zhang LY, Pan QH, Shen YY, Zhang DP (2004) Phloem unloading in developing walnut fruit is symplasmic in the seed pericarp and apoplasmic in the fleshy pericarp. Plant Cell Physiol 45:1461–1470
Zhang LY, Peng YB, Pelleschi-Travier S, Fan Y, Lu YF, Lu YM, Gao XP, Shen YY, Delrot S, Zhang DP (2004) Evidence for apoplasmic phloem unloading in developing apple fruit. Plant Physiol 135:574–586
Zhang XY, Wang XL, Wang XF, Xia GH, Pan QH, Fan RC, Wu FQ, Yu XC, Zhang DP (2006) A shift of phloem unloading from symplasmic to apoplasmic pathway is involved in developmental onset of ripening in grape berry. Plant Physiol 142:220–232
Zhou Y, Qu H, Dibley KE, Offler CE, Patrick JW (2007) A suite of sucrose transporters expressed in coats of developing legume seeds includes novel pH-independent facilitators. Plant J 49:750–764
Acknowledgments
This work was supported by funds from the Chateau Ste. Michelle Distinguished Professorship. We thank Dr. John K. Fellman for critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2013_2004_MOESM1_ESM.tif
Fig. S1 Progression of fruit ripening in Merlot and Concord grapes. Total juice solute concentration (a), pH (b), and total organic acids as the sum of all organic acids determined by HPLC (c). Samples were grouped based on visual appearance of the berry skin (1 = green; 2 = blush/pink; 3 = red/purple; 4 = blue) or juice solute concentration (5 = ripe: 20-24 ºBrix; 6 = overripe: > 24 ºBrix). Data are mean ± se where se > symbol size (n = 3-18 samples of 8-12 berries) (TIFF 61691 kb)
425_2013_2004_MOESM2_ESM.tif
Fig. S2 Changes in the proton concentration in mesocarp vacuoles and apoplast of ripening Merlot and Concord grape berries. Samples were grouped based on visual appearance of the berry skin (1 = green; 2 = blush/pink; 3 = red/purple; 4 = blue) or juice solute concentration (5 = ripe: 20-24 ºBrix; 6 = overripe: > 24 ºBrix). Data are mean ± se where se > symbol size (n = 3-18 samples of 8-12 berries) (TIFF 19305 kb)
425_2013_2004_MOESM3_ESM.tif
Fig. S3 Relationship between total vacuolar solutes and vacuolar and apoplastic hexoses (a), malate (b), and K+ (c) in ripening Merlot and Concord grape berries. Correlation coefficients are as follows: Concord apoplast, r = 0.97, vacuole, r > 0.99; Merlot apoplast, r = 0.93, vacuole, r > 0.99 (a); Concord apoplast, r = -0.67, vacuole, r = -0.73; Merlot apoplast, r = 0.35, vacuole, r = -0.79 (b); Concord apoplast, r = -0.75, vacuole, r = 0.75; Merlot apoplast, r = 0.74, vacuole, r = 0.87 (c); all P < 0.001, n ≥ 60; curves were fitted using the distance-weighted least squares method (TIFF 50133 kb)
425_2013_2004_MOESM4_ESM.tif
Fig. S4 Changes in the concentrations of oxalate (a), succinate (b), and citrate (c) in mesocarp vacuoles and apoplast of ripening Merlot and Concord grape berries. Samples were grouped based on visual appearance of the berry skin (1 = green; 2 = blush/pink; 3 = red/purple; 4 = blue) or juice solute concentration (5 = ripe: 20-24 ºBrix; 6 = overripe: > 24 ºBrix). Data are mean ± se where se > symbol size (n = 3-18 samples of 8-12 berries) (TIFF 50151 kb)
Rights and permissions
About this article
Cite this article
Keller, M., Shrestha, P.M. Solute accumulation differs in the vacuoles and apoplast of ripening grape berries. Planta 239, 633–642 (2014). https://doi.org/10.1007/s00425-013-2004-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-013-2004-z