Abstract
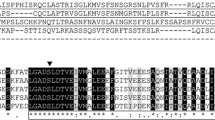
The substrate specificity of the acyl–acyl carrier protein (ACP) thioesterases significantly determines the type of fatty acids that are exported from plastids. Thus, designing acyl-ACP thioesterases with different substrate specificities or kinetic properties would be of interest for plant lipid biotechnology to produce oils enriched in specialty fatty acids. In the present work, the FatA thioesterase from Helianthus annuus was used to test the impact of changes in the amino acids present in the binding pocket on substrate specificity and catalytic efficiency. Amongst all the mutated enzymes studied, Q215W was especially interesting as it had higher specificity towards saturated acyl-ACP substrates and higher catalytic efficiency compared to wild-type H. annuus FatA. Null, wild type and high-efficiency alleles were transiently expressed in tobacco leaves to check their effect on lipid biosynthesis. Expression of active FatA thioesterases altered the composition of leaf triacylglycerols but did not alter total lipid content. However, the expression of the wild type and the high-efficiency alleles in Arabidopsis thaliana transgenic seeds resulted in a strong reduction in oil content and an increase in total saturated fatty acid content. The role and influence of acyl-ACP thioesterases in plant metabolism and their possible applications in lipid biotechnology are discussed.








Similar content being viewed by others
Abbreviations
- ACP:
-
Acyl carrier protein
- CoA:
-
Coenzyme A
- DAG:
-
Diacylglycerol
- IMAC:
-
Immobilized metal affinity chromatography
- TAG:
-
Triacylglycerides
References
Bates PA, Kelley LA, MacCallum RM, Sternberg MJE (2001) Enhancement of protein modeling by human intervention in applying the automatic programs 3D-JIGSAW and 3D-PSSM. Proteins 45:39–46
Blatti JL, Beld J, Behnke CA, Mendez M, Mayfield SP, Burkart MD (2012) Manipulating fatty acid biosynthesis in microalgae for biofuel through protein–protein interactions. PLoS One 7:e42949
Bonaventure G, Salas JJ, Pollard MR, Ohlrogge JB (2003) Disruption of the FATB gene in Arabidopsis demonstrates an essential role of saturated fatty acids in plant growth. Plant Cell 15:1020–1033
Bonaventure G, Bao X, Ohlrogge J, Pollard M (2004) Metabolic responses to the reduction in palmitate caused by disruption of the FATB gene in Arabidopsis. Plant Physiol 135:1269–1279
Bouvier-Navé P, Benveniste P, Oelkers P, Sturley SL, Schaller H (2000) Expression in yeast and tobacco of plant cDNAs encoding acyl CoA:diacylglycerol acyltransferase. Eur J Biochem 267:85–96
Burgal J, Shockey J, Lu C, Dyer J, Larson T, Graham I, Browse J (2008) Metabolic engineering of hydroxy fatty acid production in plants: rcDGAT2 drives dramatic increases in ricinoleate levels in seed oil. Plant Biotechnol J 6:819–831
Cahoon EB, Shanklin J (2000) Substrate-dependent mutant complementation to select fatty acid desaturase variants for metabolic engineering of plant seed oils. Proc Natl Acad Sci USA 97:12350–12355
Cahoon EB, Salehuzzaman S, Shanklin J, Browse J (1998) A determinant of substrate specificity predicted from the acyl–acyl carrier protein desaturase of developing cat’s claw seed. Plant Physiol 117:593–598
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Cuff JA, Barton GJ (2000) Application of enhanced multiple sequence alignment profiles to improve protein secondary structure prediction. Proteins Struct Funct Genet 40:502–511
Dehesh K, Jones A, Knutzon DS, Voelker TA (1996) Production of high levels of 8:0 and 10:0 fatty acids in transgenic canola by overexpression of ChFatB2, a thioesterase cDNA from Cuphea hookeriana. Plant J 9:167–172
Dörmann P, Kridl JC, Ohlrogge JB (1994) Cloning and expression in Escherichia coli of a cDNA coding for the oleoyl-acyl carrier protein thioesterase from coriander (Coriandrum sativum L.). Biochim Biophys Acta 1212:134–136
Facciotti MT, Yuan L (1998) Molecular dissection of the plant acyl–acyl carrier protein thioesterases. Fett Lipid 100:167–172
Facciotti MT, Bertain PB, Yuan L (1999) Improved stearate phenotype in transgenic canola expressing a modified acyl–acyl carrier protein thioesterase. Nat Biotechnol 17:593–597
Filichkin SA, Slabaugh MB, Knapp SJ (2006) New FATB thioesterases from a high-laurate Cuphea species: functional and complementation analyses. Eur J Lipid Sci Technol 108:979–990
Johnson PE, Fox SR, Hills MJ, Rawsthorne S (2000) Inhibition by long-chain acyl-CoAs of glucose 6-phosphate metabolism in plastids isolated from developing embryos of oilseed rape (Brassica napus L.). Biochem J 348:145–150
Jones A, Davies HM, Voelker TA (1995) Palmitoyl-acyl carrier protein (ACP) thioesterase and the evolutionary origin of plant acyl-ACP thioesterases. Plant Cell 7:359–371
Kapila J, DeRycke R, VanMontagu M, Angenon G (1997) An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci 122:101–108
Karimi M, Inzé D, Depicker A (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7:193–195
Larson TR, Graham IA (2001) A novel technique for the sensitive quantification of acyl CoA esters from plant tissues. Plant J 25:115–125
Larson TR, Edgell T, Byrne J, Dehesh K, Graham IA (2002) Acyl CoA profiles of transgenic plants that accumulate medium-chain fatty acids indicate inefficient storage lipid synthesis in developing oilseeds. Plant J 32:519–527
Li L, Li H, Li Q, Yang X, Zheng D, Warburton M, Chai Y, Zhang P, Guo Y, Yan J, Li J (2011) 11-bp insertion in Zea mays fatb reduces the palmitic acid content of fatty acids in maize grain. PLoS One 6:e24699
Lindqvist Y, Huang W, Schneider G, Shanklin J (1996) Crystal structure of Δ9 stearoyl-acyl carrier protein desaturase from castor seed and its relationship to other di-iron proteins. EMBO J 15:4081–4092
Mayer KM, Shanklin J (2005) A structural model of the plant acyl–acyl carrier protein thioesterase FatB comprises two helix/4-stranded sheet domains, the N-terminal domain containing residues that affect specificity and the C-terminal domain containing catalytic residues. J Biol Chem 280:3621–3627
Mayer KM, Shanklin J (2007) Identification of amino acid residues involved in substrate specificity of plant acyl-ACP thioesterases using a bioinformatics-guided approach. BMC Plant Biol 7:1
McGuffin LJ, Bryson K, Jones DT (2000) The PSIPRED protein structure prediction server. Bioinformatics 16:404–405
Moreno-Pérez AJ, Sánchez-García A, Salas JJ, Garcés R, Martínez-Force E (2011) Acyl-ACP thioesterases from macadamia (Macadamia tetraphylla) nuts: cloning, characterization and their impact on oil composition. Plant Physiol Biochem 49:82–87
Nikolau BJ, Perera MADN, Brachova L, Shanks B (2008) Platform biochemicals for a biorenewable chemical industry. Plant J 54:536–545
Ohlrogge JB (1994) Design of new plant products: engineering of fatty acid metabolism. Plant Physiol 104:821–826
Ohlrogge JB, Browse J (1995) Lipid biosynthesis. Plant Cell 7:957–970
Ohlrogge JB, Jaworski JG (1997) Regulation of fatty acid synthesis. Annu Rev Plant Physiol Plant Mol Biol 48:109–136
Ouali M, King RD (2000) Cascaded multiple classifiers for secondary structure prediction. Prot Sci 9:1162–1176
Pollastri G, Przybylski D, Rost B, Baldi P (2002) Improving the prediction of protein secondary structure in three and eight classes using recurrent neural networks and profiles. Proteins 47:228–235
Rawsthorne S (2002) Carbon flux and fatty acid synthesis in plants. Prog Lipid Res 41:182–196
Rock CO, Garwin JL (1979) Preparative enzymatic synthesis and hydrophobic chromatography of acyl–acyl carrier protein. J Biol Chem 254:7123–7128
Rost B (1996) PHD: predicting one-dimensional protein structure by profile-based neural networks. Methods Enzymol 266:525–539
Salas JJ, Ohlrogge JB (2002) Characterization of substrate specificity of plant FatA and FatB acyl-ACP thioesterases. Arch Biochem Biophys 403:25–34
Sánchez-García A, Moreno-Pérez AJ, Muro-Pastor AM, Salas JJ, Garcés R, Martínez-Force E (2010) Acyl-ACP thioesterases from castor (Ricinus communis L.): an enzymatic system appropriate for high rates of oil synthesis and accumulation. Phytochemistry 71:860–869
Schwede T, Kopp J, Guex N, Peitsch MC (2003) SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res 31:3381–3385
Serrano-Vega MJ, Garcés R, Martínez-Force E (2005) Cloning, characterization and structural model of a FatA-type thioesterase from sunflower seeds (Helianthus annuus L.). Planta 221:868–880
Shockey JM, Fulda MS, Browse JA (2002) Arabidopsis contains nine long-chain acyl-coenzyme A synthetase genes that participate in fatty acid and glycerolipid metabolism. Plant Physiol 129:1710–1722
Voelker TA, Worrell AC, Anderson L, Bleibaum J, Fan C, Hawkins DJ, Radke SE, Davies HM (1992) Fatty acid biosynthesis redirected to medium chains in transgenic oilseed plants. Science 257:72–74
Voelker TA, Jones A, Cranmer AM, Davies HM, Knutzon DS (1997) Broad-range and binary-range acyl–acyl-carrier-protein thioesterases suggest an alternative mechanism for medium-chain production in seeds. Plant Physiol 114:669–677
Voinnet O, Rivas S, Mestre P, Baulcombe D (2003) An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J 33:949–956
Wu PZ, Li J, Wei Q, Zeng L, Chen YP, Li MR, Jiang HW, Wu GJ (2009) Cloning and functional characterization of an acyl–acyl carrier protein thioesterase (JcFATB1) from Jatropha curcas. Tree Physiol 29:1299–1305
Yuan L, Voelker TA, Hawkins DJ (1995) Modification of the substrate specificity of an acyl–acyl carrier protein thioesterase by protein engineering. Proc Natl Acad Sci USA 92:10639–10643
Yuan L, Nelson BA, Caryl G (1996) The catalytic cysteine and histidine in the plant acyl–acyl carrier protein thioesterases. J Biol Chem 271:3417–3419
Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JDG, Boller T, Felix G (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125:749–760
Acknowledgments
We are grateful to Rosario Sánchez and Valeria Gazda for their technical assistance. This work was supported by the MINECO and FEDER (Project AGL2011-23187).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moreno-Pérez, A.J., Venegas-Calerón, M., Vaistij, F.E. et al. Effect of a mutagenized acyl-ACP thioesterase FATA allele from sunflower with improved activity in tobacco leaves and Arabidopsis seeds. Planta 239, 667–677 (2014). https://doi.org/10.1007/s00425-013-2003-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-013-2003-0