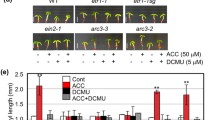
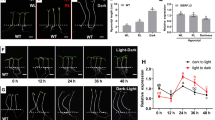
Abstract
Ethylene and light act through specific signal transduction mechanisms to coordinate the development of higher plants. Application of 1-aminocyclopropane-1-carboxylic acid (ACC, an ethylene precursor) suppresses the hypocotyl elongation of Arabidopsis seedlings in dark, but stimulates it in light. However, the mechanisms of opposite effects of ethylene on hypocotyl elongation in light and dark remain unclear. In the present study, we investigated the key factors involved in the opposite effects of ethylene on hypocotyl elongation in Arabidopsis seedlings. The effects of ACC on hypocotyl elongation of IAA-insensitive mutants including tir1-1, axr1-3, and axr1-12 seedlings were reduced in light but not in dark. The DR5 promoter, a synthetic auxin-response promoter, was used to quantify the level of IAA responses. There was a marked increase in DR5-GFP signals in response to ACC treatment in hypocotyls of DR5-GFP seedlings in light, but not in dark. CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) is an important downstream component of light signaling. ETHYLENE-INSENSITIVE3 (EIN3, an ethylene-stabilized transcription factor) directly regulates ETHYLENE-RESPONSE-FACTOR1 (ERF1). The cop1-4 mutant treated with ACC and cop1-4/EIN3ox plants developed long hypocotyls in darkness. Expression of ERF1 in the cop1-4 mutant was induced by ACC treatment in dark, but the expression of ERF1 in the wild type was not affected. Taken together, ethylene-promoting hypocotyl via IAA is mediated by light, and COP1 has a significant impact on the transcription of some genes downstream of EIN3. Thus, COP1 plays a crucial role in the opposite effects of ethylene on hypocotyl elongation.









Similar content being viewed by others
Abbreviations
- ACC:
-
1-Aminocyclopropane-1-carboxylic acid
- COP1:
-
CONSTITUTIVELY PHOTOMORPHOGENIC 1
- HY5:
-
LONG HYPOCOTYL 5
- LMN:
-
Low nutrient medium
- EIN3:
-
ETHYLENE-INSENSITIVE3
- ERF1:
-
ETHYLENE-RESPONSE-FACTOR 1
- IAA:
-
Indole-3-acetic acid
- NPA:
-
1-N-naphthylphthalamic acid
- PIF1:
-
PHYTOCHROME INTERACTING FACTOR 1
References
Alabadi D, Gallego-Bartolome J, Orlando L, Garcia-Carcel L, Rubio V, Martinez C, Frigerio M, Iglesias-Pedraz JM, Espinosa A, Deng XW (2008) Gibberellins modulate light signaling pathways to prevent Arabidopsis seedling de-etiolation in darkness. Plant J 53:324–335
An F, Zhao Q, Ji Y, Li W, Jiang Z, Yu X, Zhang C, Han Y, He W, Liu Y (2010) Ethylene-induced stabilization of ETHYLENE INSENSITIVE3 and EIN3-LIKE1 is mediated by proteasomal degradation of EIN3 binding F-box 1 and 2 that requires EIN2 in Arabidopsis. Plant Cell 22:2384–2401
Ang LH, Deng XW (1994) Regulatory hierarchy of photomorphogenic loci: allele-specific and light-dependent interaction between the HY5 and COP1 loci. Plant Cell 6:613–628
Boerjan W, Cervera MT, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Onckelen HV, Montagu MV, Inzé D (1995) Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7:1405–1419
Chattopadhyay S, Ang LH, Puente P, Deng XW, Wei N (1998) Arabidopsis bZIP protein HY5 directly interacts with light-responsive promoters in mediating light control of gene expression. Plant Cell 10:673–684
Chen YF, Etheridge N, Schaller G (2005) Ethylene signal transduction. Ann Bot 95:901–915
Chen H, Xue L, Chintamanani S, Germain H, Lin H, Cui H, Cai R, Zuo J, Tang X, Li X (2009) ETHYLENE INSENSITIVE3 and ETHYLENE INSENSITIVE3-LIKE1 repress SALICYLIC ACID INDUCTION DEFICIENT2 expression to negatively regulate plant innate immunity in Arabidopsis. Plant Cell 21:2527–2540
Cheng Y, Dai X, Zhao Y (2006) Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev 20:1790–1799
Cheng Y, Dai X, Zhao Y (2007) Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 19:2430–2439
Collett CE, Harberd NP, Leyser O (2000) Hormonal interactions in the control of Arabidopsis hypocotyl elongation. Plant Physiol 124:553–562
Davies PJ (1995) Plant hormones: Physiology, biochemistry and molecular biology. Kluwer Academic Publishers, New York
Feng S, Martinez C, Gusmaroli G, Wang Y, Zhou J, Wang F, Chen L, Yu L, Iglesias-Pedraz JM, Kircher S (2008) Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451:475–479
Fujimoto SY, Ohta M, Usui A, Shinshi H, Ohme-Takagi M (2000) Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell 12:393–404
Gray WM, Östin A, Sandberg G, Romano CP, Estelle M (1998) High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc Natl Acad Sci USA 95:7197–7202
Guo H, Ecker JR (2003) Plant responses to ethylene gas are mediated by SCFEBF1/EBF2-dependent proteolysis of EIN3 transcription factor. Cell 115:667–677
Holm M, Ma LG, Qu LJ, Deng XW (2002) Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev 16:1247–1259
Jensen PJ, Hangarter RP, Estelle M (1998) Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiol 116:455–462
Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Tongprasit W, Zhao H, Lee I, Deng XW (2007) Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19:731–749
Lehman A, Black R, Ecker JR (1996) HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell 85:183–194
Li H, Guo H (2007) Molecular basis of the ethylene signaling and response pathway in Arabidopsis. J Plant Growth Regul 26:106–117
Li H, Johnson P, Stepanova A, Alonso JM, Ecker JR (2004) Convergence of signaling pathways in the control of differential cell growth in Arabidopsis. Dev Cell 7:193–204
Ma L, Li J, Qu L, Hager J, Chen Z, Zhao H, Deng XW (2001) Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell 13:2589–2607
Ma L, Gao Y, Qu L, Chen Z, Li J, Zhao H, Deng XW (2002) Genomic evidence for COP1 as a repressor of light-regulated gene expression and development in Arabidopsis. Plant Cell 14:2383–2398
McNellis TW, Arnim AG, Deng XW (1994) Overexpression of Arabidopsis COP1 results in partial suppression of light-mediated development: evidence for a light-inactivable repressor of photomorphogenesis. Plant Cell 6:1391–1400
Oono Y, Chen QG, Overvoorde PJ, Köhler C, Theologis A (1998) Age mutants of Arabidopsis exhibit altered auxin-regulated gene expression. Plant Cell 10:1649–1662
Osterlund MT, Ang LH, Deng XW (1999) The role of COP1 in repression of Arabidopsis photomorphogenic development. Trends Cell Biol 9:113–118
Osterlund MT, Hardtke CS, Wei N, Deng XW (2000) Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405:462–466
Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P (2003) EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell 115:679–689
Romano CP, Robson PRH, Smith H, Estelle M, Klee H (1995) Transgene-mediated auxin overproduction in Arabidopsis: hypocotyl elongation phenotype and interactions with the hy6-1 hypocotyl elongation and axr1 auxin-resistant mutants. Plant Mol Biol 27:1071–1083
Ruzicka K, Ljung K, Vanneste S, Podhorska R, Beeckman T, Friml J, Benkova E (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19:2197–2212
Saibo NJM, Vriezen WH, Beemster GTS, Van Der Straeten D (2003) Growth and stomata development of Arabidopsis hypocotyls are controlled by gibberellins and modulated by ethylene and auxins. Plant J 33:989–1000
Schwechheimer C, Deng XW (2000) The COP/DET/FUS proteins—regulators of eukaryotic growth and development. Semin Cell Dev Biol 11:495–503
Smalle J, Haegman M, Kurepa J, Van Montagu M, Straeten DVD (1997) Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA 94:2756–2761
Smith H (2000) Phytochromes and light signal perception by plants—an emerging synthesis. Nature 407:585–591
Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12:3703–3714
Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jurgens G, Alonso JM (2008) TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133:177–191
Stepanova AN, Yun J, Robles LM, Novak O, He W, Guo H, Ljung K, Alonso JM (2011) The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis. Plant Cell 23:3961–3973
Sullivan JA, Shirasu K, Deng XW (2003) The diverse roles of ubiquitin and the 26S proteasome in the life of plants. Nat Rev Genet 4:948–958
Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GT, Sandberg G, Bhalerao R, Ljung K, Bennett MJ (2007) Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 19:2186–2196
Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9:1963–1971
Vandenbussche F, Smalle J, Le J, Saibo NJM, De Paepe A, Chaerle L, Tietz O, Smets R, Laarhoven LJJ, Harren FJM (2003) The Arabidopsis mutant alh1 illustrates a cross talk between ethylene and auxin. Plant Physiol 131:1228–1238
Vandenbussche F, Vancompernolle B, Rieu I, Ahmad M, Phillips A, Moritz T, Hedden P, Van Der Straeten D (2007) Ethylene-induced Arabidopsis hypocotyl elongation is dependent on but not mediated by gibberellins. J Exp Bot 58:4269–4281
Vandenbussche F, Petrasek J, Zadnikova P, Hoyerova K, Pesek B, Raz V, Swarup R, Bennett M, Zazimalova E, Benkova E, Van Der Straeten D (2010) The auxin influx carriers AUX1 and LAX3 are involved in auxin–ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development 137:597–606
von Arnim AG, Deng XW (1994) Light inactivation of Arabidopsis photomorphogenic repressor COP1 involves a cell-specific regulation of its nucleocytoplasmic partitioning. Cell 79:1035–1045
Yamamoto YY, Matsui M, Ang LH, Deng XW (1998) Role of a COP1 interactive protein in mediating light-regulated gene expression in Arabidopsis. Plant Cell 10:1083–1094
Yamamoto Y, Kamiya N, Morinaka Y, Matsuoka M, Sazuka T (2007) Auxin biosynthesis by the YUCCA genes in rice. Plant Physiol 143:1362–1371
Zadnikova P, Petrasek J, Marhavy P, Raz V, Vandenbussche F, Ding Z, Schwarzerova K, Morita MT, Tasaka M, Hejatko J, Van Der Straeten D, Friml J, Benkova E (2010) Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana. Development 137:607–617
Zhao Y (2010) Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol 61:49–64
Zhong S, Zhao M, Shi T, Shi H, An F, Zhao Q, Guo H (2009) EIN3/EIL1 cooperate with PIF1 to prevent photo-oxidation and to promote greening of Arabidopsis seedlings. Proc Natl Acad Sci USA 106:21431–21436
Zhu Z, Guo H (2008) Genetic basis of ethylene perception and signal transduction in Arabidopsis. J Integr Plant Biol 50:808–815
Acknowledgments
We would like to thank Professor Hongwei Guo (Peking University) for providing Arabidopsis seeds of cop1-4/EIN3ox and EIN3ox mutants, and Professor Xingwang Deng (Peking University) for providing Arabidopsis seeds of cop1-4, hy5, hy5hyh, and hyh mutants used in this study. This work was supported by the National Natural Science Foundation of China (31170225), the Doctoral Program of Higher Education of China (ratification number: 20100211110009), Foundation of Science and Technology Program of Gansu Province (1107RJYA005), and the Fundamental Research Funds for the Central Universities (lzujbky-2012-104).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liang, X., Wang, H., Mao, L. et al. Involvement of COP1 in ethylene- and light-regulated hypocotyl elongation. Planta 236, 1791–1802 (2012). https://doi.org/10.1007/s00425-012-1730-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-012-1730-y