Abstract
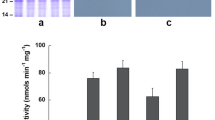
Soybeans provide an excellent source of protein in animal feed. Soybean protein quality can be enhanced by increasing the concentration of sulfur-containing amino acids. Previous attempts to increase the concentration of sulfur-containing amino acids through the expression of heterologous proteins have met with limited success. Here, we report a successful strategy to increase the cysteine content of soybean seed through the overexpression of a key sulfur assimilatory enzyme. We have generated several transgenic soybean plants that overexpress a cytosolic isoform of O-acetylserine sulfhydrylase (OASS). These transgenic soybean plants exhibit a four- to tenfold increase in OASS activity when compared with non-transformed wild-type. The OASS activity in the transgenic soybeans was significantly higher at all the stages of seed development. Unlike the non-transformed soybean plants, there was no marked decrease in the OASS activity even at later stages of seed development. Overexpression of cytosolic OASS resulted in a 58–74% increase in protein-bound cysteine levels compared with non-transformed wild-type soybean seeds. A 22–32% increase in the free cysteine levels was also observed in transgenic soybeans overexpressing OASS. Furthermore, these transgenic soybean plants showed a marked increase in the accumulation of Bowman–Birk protease inhibitor, a cysteine-rich protein. The overall increase in soybean total cysteine content (both free and protein-bound) satisfies the recommended levels required for the optimal growth of monogastric animals.






Similar content being viewed by others
Abbreviations
- OASS:
-
O-acetylserine sulfhydrylase
- SAT:
-
Serine acetyltransferase
- HSD:
-
Homoserine dehydrogenase
References
Amir R, Galili G (2003) Approaches to improve the nutritional values of transgenic plants by increasing their methionine content. In: Hemantaranjan A (ed) Advances in plant physiology 6. Scientific Publishers, Jodhpur, pp 61–77
Amir R, Tabe L (2006) Molecular approaches to improving plant methionine content. In: Pawan KJ, Rana PS (eds) Plant genetic engineering. Metabolic engineering and molecular farming II, vol. 8. Studium Press LLC, pp 1–26
Avraham T, Badani H, Galili S, Amir R (2005) Enhanced levels of methionine and cysteine in transgenic alfalfa (Medicago sativa L.) plants over-expressing the Arabidopsis cystathionine γ-synthase gene. Plant Biotechnol J 3:71–79
Birk Y (1985) The Bowman–Birk inhibitor. Int J Pept Protein Res 25:113–131
Blaszczyk A, Brodzik R, Sirko A (1999) Increased resistance to oxidative stress in transgenic tobacco plants overexpressing bacterial serine acetyltransferase. Plant J 20:237–243
Bogdanova N, Hell R (1997) Cysteine synthesis in plants: protein–protein interactions of serine acetyltransferase from Arabidopsis thaliana. Plant J 11:251–262
Bonner ER, Cahoon RE, Knapke SM, Jez JM (2005) Molecular basis of plant cysteine biosynthesis: structural and functional analysis of O-acetylserine sulfhydrylase from Arabidopsis thaliana. J Biol Chem 280:38803–38813
Chronis D, Krishnan HB (2003) Sulfur assimilation in soybean: molecular cloning and characterization of O-acetylserine(thiol)lyase (cysteine synthase). Crop Sci 43:1819–1827
Chronis D, Krishnan HB (2004) Sulfur assimilation in soybean (Glycine max [L.] Merr.): molecular cloning and characterization of a cytosolic isoform of serine acetyltransferase. Planta 218:417–426
Clarke EJ, Wiseman J (2000) Developments in plant breeding for improved nutritional quality of soy beans II. Anti-nutritional factors. J Ag Sci 134:125–136
Dinkins RD, Reddy MSS, Meurer CA, Yan B, Trick H, Thibaud-Nissen F, Finer JJ, Parrott WA, Collins GB (2001) Increased sulfur amino acids in soybean plants overexpressing the maize 15 kDa zein protein. In Vitro Cell Dev Biol-Plant 37:742–747
Dominguez-Solis JR, Gutierrez-Alcala G, Vega JM, Romero LC, Gotor C (2001) The cytosolic O-acetylserine(thiol)lyase gene is regulated by heavy metals and can function in cadmium tolerance. J Biol Chem 276:9297–9302
Droux M (2003) Plant serine acetyltransferase: new insights for regulation of sulphur metabolism in plant cells. Plant Physiol Biochem 41:619–627
Falco SC, Guida T, Locke M, Mauvais J, Sandres C, Ward RT, Webber P (1995) Transgenic canola and soybean seeds with increased lysine. Biotechnology 13:577–582
Francois JA, Kumaran S, Jez JM (2006) Structural basis for interaction of O-acetylserine sulfhydrylase and serine acetyltransferase in the Arabidopsis cysteine synthase complex. Plant Cell 18:3647–3655
Hacham Y, Avraham T, Amir R (2002) The N-terminal region of Arabidopsis cystathionine gamma-synthase plays an important regulatory role in methionine metabolism. Plant Physiol 128:454–462
Hagan ND, Upadhyaya N, Tabe LM, Higgins TJ (2003) The redistribution of protein sulfur in transgenic rice expressing a gene for a foreign, sulfur-rich protein. Plant J 34:1–11
Harms K, von Ballmoos P, Brunold C, Hofgen R, Hesse H (2000) Expression of a bacterial serine acetyltransferase in transgenic potato plants leads to increased levels of cysteine and glutathione. Plant J 22:335–343
Heeg C, Kruse C, Jost R, Gutensohn M, Ruppert T, Wirtz M, Hell R (2008) Analysis of the Arabidopsis O-acetylserine(thiol)lyase gene family demonstrates compartment-specific differences in the regulation of cysteine synthesis. Plant Cell 20:168–185
Hell R, Hillebrand H (2001) Plant concepts for mineral acquisition and allocation. Curr Opin Biotech 12:161–168
Hinchee MAW, Connor-Ward DV, Newell CA, McDonnell RE, Sato SJ, Gasser CS, Fischhoff DA, Re DB, Fraley RT, Horsch RB (1988) Production of transgenic soybean plants using Agrobacterium-mediated DNA transfer. Bio/Technology 6:915–922
Imsande J (2001) Selection of soybean mutants with increased concentrations of seed methionine and cysteine. Crop Sci 41:510–515
Jez JM, Krishnan HB (2009) Sulfur assimilation and cysteine biosynthesis in soybean seeds: towards engineering sulfur amino acid content. In: Krishnan HB (ed) Modification of seed composition to promote health and nutrition. ASA-CSSA-SSSA Publishing, Madison, pp 249–261
Jung R (1997) Expression of a 2S albumin from Bertholletia excelsain soybean. In: The 39th NIBB Conference: Dynamic aspects of seed maturation and germination. Okazaki: National Institute for Basic Biology. URL: http://www.pubs.nrc-cnrc.gc.ca/ispmb/isqmb15/15393-4.pdf
Kim WS, Krishnan HB (2004) Expression of an 11 kDa methionine-rich delta-zein in transgenic soybean results in the formation of two types of novel protein bodies in transitional cells situated between the vascular tissue and storage parenchyma cells. Plant Biotech J 2:199–210
Krishnan HB (2004) A simple and rapid method to isolate low molecular weight proteinase inhibitors from soybean. Korean J Crop Sci 49:342–348
Krishnan HB (2005) Engineering soybean for enhanced sulfur amino acid content. Crop Sci 45:454–461
Krishnan HB (2008) Improving the sulfur-containing amino acids of soybeans to enhance its nutritional value in animal feed. In: Jez JM (ed) Sulfur: a missing link between soils crops and nutrition. ASA-CSSA-SSSA Publishing, Madison, pp 235–249
Kumaran S, Jez JM (2007) Thermodynamics of the interaction between O-acetylserine sulfhydrylase and the C-terminus of serine acetyltransferase. Biochemistry 46:5586–5594
Kumaran S, Yi H, Krishnan HB, Jez JM (2009) Assembly of the cysteine synthase complex and the regulatory role of protein–protein interactions. J Biol Chem 284:10268–10275
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Leustek T, Martin MN, Bick JA, Davies JP (2000) Pathways and regulation of sulfur metabolism revealed through molecular and genetic studies. Annu Rev Plant Physiol Plant Mol Biol 51:141–165
Liu F, Yoo BC, Lee JY, Pan W, Harmon AC (2006) Calcium-regulated phosphorylation of soybean serine acetyltransferase in response to oxidative stress. J Biol Chem 281:27405–27415
Lopez-Martin MC, Becana M, Romero LC, Gotor C (2008) Knocking out cytosolic cysteine synthesis compromises the antioxidant capacity of the cytosol to maintain discrete concentrations of hydrogen peroxide in Arabidopsis. Plant Physiol 147:562–572
Mason G, Provero P, Vaira AM, Accotto GP (2002) Estimating the number of integrations in transformed plants by quantitative real-time PCR. BMC Biotechnology 2:20
Müntz K, Christov V, Saalbach G, Saalbach I, Waddell D, Pickardt T, Schieder O, Wustenhagen T (1998) Genetic engineering for high methionine grain legumes. Nahrung 42:125–137
Nielsen NC (1996) Soybean seed composition. In: Verma DPS, Shoemaker RC (eds) Soybean: genetics molecular biology and biotechnology. CAB, Wallingford, pp 127–163
Ning H, Zhang C, Yao Y, Yu D (2010) Overexpression of a soybean O-acetylserine (thiol) lyase-encoding gene GmOASTL4 in tobacco increases cysteine levels and enhances tolerance to cadmium stress. Biotechnol Lett 32:557–564
Noji M, Saito K (2002) Molecular and biochemical analysis of serine acetyltransferase and cysteine synthase towards sulfur metabolism engineering. Amino Acids 22:231–243
Noji M, Saito M, Nakamura M, Aono M, Saji H, Saito K (2001) Cysteine synthase overexpression in tobacco confers tolerance to sulfur-containing environmental pollutants. Plant Physiol 126:973–980
Omar AA, Dekkers MG, Graham JH, Grosser JW (2008) Estimation of transgene copy number in transformed citrus plants by quantitative multiplex real-time PCR. Biotechnol Prog 24:1241–1248
Qi Q, Huang J, Crowley J, Ruschke L, Goldman BS, Wen L, Rapp WD (2011) Metabolically engineered soybean seed with enhanced threonine levels: biochemical characterization and seed-specific expression of lysine-insensitive variants of aspartate kinases from the enteric bacterium Xenorhabdus bovienii. Plant Biotechnol J 9:193–204
Ruffet ML, Lebrun M, Droux M, Douce R (1995) Subcellular distribution of serine acetyltransferase from Pisum sativum and characterization of an Arabidopsis thaliana putative cytosolic isoform. Eur J Biochem 227:500–509
Saito K (2000) Regulation of sulfate transport and synthesis of sulfur-containing amino acids. Curr Opin Plant Biol 3:188–195
Schroeder AC, Zhu C, Yanamadala SR, Cahoon RE, Arkus KA, Wachsstock L, Bleeke J, Krishnan HB, Jez JM (2010) Threonine-insensitive homoserine dehydrogenase from soybean: genomic organization, kinetic mechanism, and in vivo activity. J Biol Chem 285:827–834
Shewry PR (2000) Seed proteins. In: Black M, Bewley JD (eds) Seed technology and its biological basis. Sheffield Academic Press, Sheffield, pp 42–84
Sirko A, Blaszczyk A, Liszewska F (2004) Overproduction of SAT and/or OASTL in transgenic plants: a survey of effects. J Exp Bot 55:1881–1888
Streit LG, Beach LR, Register JC, Jung R, Fehr WR (2001) Association of the Brazil nut protein gene and Kunitz trypsin inhibitor alleles with soybean protease inhibitor activity and agronomic traits. Crop Sci 41:1757–1760
Tabe LM, Droux M (2002) Limits to sulfur accumulation in transgenic lupin seeds expressing a foreign sulfur-rich protein. Plant Physiol 128:1137–1148
Tabe L, Higgins TJ (1998) Engineering plant protein composition for improved nutrition. Trends Plant Sci 3:282–286
Tabe L, Wirtz M, Molvig L, Droux M, Hell R (2010) Overexpression of serine acetyltransferase produced large increases in O-acetylserine and free cysteine in developing seeds of grain legume. J Exp Bot 61:721–733
Townsend JA, Thomas LA (1994) Factors which influence the Agrobacterium-mediated transformation of soybean. J Cell Biochem 56, Suppl. 18A:78
Ufaz S, Galili G (2008) Improving the content of essential amino acids in crop plants: goals and opportunities. Plant Physiol 147:954–961
Warrilow AGS, Hawkesford MJ (1998) Separation, subecellular location and influence of sulphur nutrition on isoforms of cysteine synthase in spinach. J Exp Bot 49:1625–1636
Watanabe M, Kusano M, Oikawa A, Fukushima A, Noji M, Saito K (2008) Physiological roles of the beta-substituted alanine synthase gene family in Arabidopsis. Plant Physiol 146:310–320
Wirtz M, Hell R (2003) Production of cysteine for bacterial and plant biotechnology: application of cysteine feedback-insensitive isoforms of serine acetyltransferase. Amino Acids 24:195–203
Yi H, Galant A, Ravilious GE, Preuss ML, Jez JM (2010a) Sensing sulfur conditions: simple to complex biochemical regulatory mechanisms in plant thiol metabolism. Mol Plant 3:269–279
Yi H, Ravilious GE, Galant A, Krishnan HB, Jez JM (2010b) From sulfur to homoglutathione: thiol metabolism in soybean. Amino Acids 39:963–978
Youssefian S, Nakamura M, Orudgev E, Kondo N (2001) Increased cysteine biosynthesis capacity of transgenic tobacco overexpressing an O-acetylserine(thiol) lyase modifies plant responses to oxidative stress. Plant Physiol 126:1001–1011
Zhang Z, Xing A, Staswick P, Clemente TE (1999) The use of glufosinate as a selective agent in Agrobacterium-mediated transformation of soybean. Plant Cell Tissue Organ Cult 56:37–46
Zhang C, Meng Q, Gai J, Yu D (2008a) Cloning and functional characterization of an O-acetylserine(thiol)lyase-encoding gene in wild soybean (Glycine soja). Mol Biol Rep 35:527–534
Zhang C, Meng Q, Zhang M, Huang F, Gai J, Yu D (2008b) Characterization of O-acetylserine(thiol)lyase-encoding genes reveals their distinct but cooperative expression in cysteine synthesis of soybean [Glycine max (L.) Merr.]. Plant Mol Biol Rep 26:277–291
Acknowledgments
We thank the University of Missouri Plant Transformation Core Facility for the production of transgenic soybean lines. Work in the Jez lab was funded by a US Department of Agriculture grant (NRI-2005-02518) to J.M.J., M.J. and A.C.S. were also supported in part by American Society of Plant Biologists Summer Undergraduate Research Fellowships. Product names are necessary to report factually on available data; however, the University of Missouri and the USDA neither guarantee nor warrant the standard of product, and the use of the name by the University of Missouri and the USDA implies no approval of the product to the exclusion of others that may be suitable.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, WS., Chronis, D., Juergens, M. et al. Transgenic soybean plants overexpressing O-acetylserine sulfhydrylase accumulate enhanced levels of cysteine and Bowman–Birk protease inhibitor in seeds. Planta 235, 13–23 (2012). https://doi.org/10.1007/s00425-011-1487-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-011-1487-8