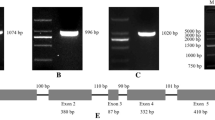
Abstract
Plasma membrane proton pumps (PM H+-ATPases) are involved in several physiological processes, such as growth and development, and abiotic stress responses. The major regulators of the PM H+-ATPases are proteins of the 14-3-3 family, which stimulate its activity. In addition, a novel interaction partner of the AHA1 PM H+-ATPase, named PPI1 (proton pump interactor, isoform 1), was identified in Arabidopsis thaliana. This protein stimulates the activity of the proton pump in vitro. In this work, we report the characterization of an A. thaliana PPI1 homolog in potato (Solanum tuberosum L.) named StPPI1. The full-length coding sequence of StPPI1 was obtained. The open reading frame (ORF) encodes a protein of 629 amino acids showing 50% identity with A. thaliana PPI1 protein. The StPPI1 ORF is divided into seven exons split by six introns. Southern blot analysis suggests that StPPI1 belongs to a family of related genes. Recombinant StPPI1 stimulates H+-ATPase activity in vitro. Basal levels of StPPI1 transcripts are observed in all tissues, however, StPPI1 expression is higher in proliferative regions (shoot apex and flower buds), flowers and leaves than in shoots and roots. StPPI1 mRNA levels significantly increase during tuber development. StPPI1 is induced by salt stress and cold. Drought and mechanical wounding slightly increase StPPI1 transcript levels. In addition, the expression of SlPPI1, the tomato homolog of StPPI1, was determined under adverse environmental conditions in tomato plants. SlPPI1 mRNA levels are increased by drought and cold, but are unaffected by salt stress. Mechanical wounding slightly increases SlPPI1 expression.








Similar content being viewed by others
Abbreviations
- PPI:
-
Proton pump interactor
- PM:
-
Plasma membrane
- ABA:
-
Abscisic acid
References
Anzi C, Pelucchi P, Vazzola V, Murgia I, Gomarasca S, Piccoli MB, Morandini P (2008) The proton pump interactor (Ppi) gene family of Arabidopsis thaliana: expression pattern of Ppi1 and characterisation of knockout mutants for Ppi1 and 2. Plant Biol (Stuttg) 10:237–249
Arango M, Gévaudant F, Oufattole M, Boutry M (2003) The plasma membrane proton pump ATPase: the significance of gene subfamilies. Planta 216:355–365
Baruah KK, Alyoshina NV, Astakhova NB, Trunova TI (1990) Characterization of plasma membrane of winter wheat (Triticum aestivum L.) seedlings and changes in ATP hydrolyzing activity under low temperature stress. Biol Plant 32:211–217
Baxter I, Tchieu J, Sussman MR, Boutry M, Palmgren MG, Gribskov M, Harper JF, Axelsen KB (2003) Genomic comparison of P-type ATPase ion pumps in Arabidopsis and rice. Plant Physiol 132:618–628
Bonza MC, Fusca T, Homann U, Thiel G, De Michelis MI (2009) Intracellular localisation of PPI1 (proton pump interactor, isoform 1), a regulatory protein of the plasma membrane H+-ATPase of Arabidopsis thaliana. Plant Biol (Stuttg) 11:869–877
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Büchter R, Strömberg A, Schmelzer E, Kombrink E (1997) Primary structure and expression of acidic (class II) chitinase in potato. Plant Mol Biol 35:749–761
Camoni L, Iori V, Marra M, Aducci P (2000) Phosphorylation-dependent interaction between plant plasma membrane H+-ATPase and 14-3-3 proteins. J Biol Chem 275:9919–9923
Capiati DA, País SM, Téllez-Iñón MT (2006) Wounding increases salt tolerance in tomato plants: evidence on the participation of calmodulin-like activities in cross-tolerance signalling. J Exp Bot 57:2391–2400
Chelysheva VV, Smolenskaya IN, Trofimova MC, Babakov AV, Muromtsev GS (1999) Role of the 14-3-3 proteins in the regulation of H+-ATPase activity in the plasma membrane of suspension-cultured sugar beet cells under cold stress. FEBS Lett 456:22–26
Chen Z, Pottosin II, Cuin TA, Fuglsang AT, Tester M, Jha D, Zepeda-Jazo I, Zhou M, Palmgren MG, Newman IA, Shabala S (2007) Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiol 145:1714–1725
Duby G, Poreba W, Piotrowiak D, Bobik K, Derua R, Waelkens E, Boutry M (2009) Activation of plant plasma membrane H+-ATPase by 14-3-3 proteins is negatively controlled by two phosphorylation sites within the H+-ATPase C-terminal region. J Biol Chem 284:4213–4221
Ferl RJ (2004) 14-3-3 proteins: regulation of signal-induced events. Physiol Plant 120:173–178
Fuglsang AT, Visconti S, Drumm K, Jahn T, Stensballe A, Mattei B, Jensen ON, Aducci P, Palmgren MG (1999) Binding of 14-3-3 protein to the plasma membrane H+-ATPase AHA2 involves the three C-terminal residues Tyr(946)-Thr-Val and requires phosphorylation of Thr(947). J Biol Chem 274:36774–36780
Gaxiola RA, Palmgren MG, Schumacher K (2007) Plant proton pumps. FEBS Lett 581:2204–2214
Gévaudant F, Duby G, von Stedingk E, Zhao R, Morsomme P, Boutry M (2007) Expression of a constitutively activated plasma membrane H+-ATPase alters plant development and increases salt tolerance. Plant Physiol 144:1763–1776
Godoy JA, Pardo JM, Pintor-Toro JA (1990) A tomato cDNA inducible by salt stress and abscisic acid: nucleotide sequence and expression pattern. Plant Mol Biol 26:1921–1934
Harms K, Wohner RV, Schulz B, Frommer WB (1994) Isolation and characterization of P-type H+-ATPase genes from potato. Plant Mol Biol 26:979–988
Hebsgaard SM, Korning PG, Tolstrup N, Engelbrecht J, Rouze P, Brunak S (1996) Splice site prediction in Arabidopsis thaliana DNA by combining local and global sequence information. Nucleic Acids Res 24:3439–3452
Hellergren J, Widell S, Lundborg T, Kylin A (1983) Frost hardiness development in Pinus sylvestris: the involvement of a K+-stimulated Mg2+-dependent ATPase from purified plasma membranes of pine. Physiol Plant 58:7–12
Hiribayashi S, Matsushita Y, Sato M, Ohi R, Kasahara M, Abe H, Nyunoya H (2004) Two proton pump interactors identified from a direct phosphorylation screening of a rice cDNA library by using a recombinant BRI1 receptor kinase. Plant Biotechnol 21:35–45
Hohenwallner W, Wimmer E (1973) Malachite green micromethod for determination of inorganic-phosphate. Clin Chim Acta 45:169–175
Jahn T, Fuglsang AT, Olsson A, Bruntrup IM, Collinge DB, Volkmann D, Sommarin M, Palmgren MG, Larsson C (1997) The 14-3-3 protein interacts directly with the C-terminal region of the plant plasma membrane H+-ATPase. Plant Cell 9:1805–1814
Janicka-Russak M, Klobus G (2007) Modification of plasma membrane and vacuolar H+-ATPases in response to NaCl and ABA. J Plant Physiol 164:295–302
Johansson F, Olbe M, Sommarin M, Larsson C (1995) Brij 58, a polyoxyethylene acyl ether, creates membrane vesicles of uniform sidedness. A new tool to obtain inside-out (cytoplasmic side-out) plasma membrane vesicles. Plant J 7:165–173
Kalampanayil BD, Wimmers LE (2001) Identification and characterization of a salt-stress-induced plasma membrane H+-ATPase in tomato. Plant Cell Environ 24:999–1000
López-Pérez L, Martínez-Ballesta MC, Maurel C, Carvajal M (2009) Changes in plasma membrane lipids, aquaporins and proton pump of broccoli roots, as an adaptation mechanism to salinity. Phytochemistry 70:492–500
Martz F, Sutinen ML, Kiviniemi S, Palta JP (2006) Changes in freezing tolerance, plasma membrane H+-ATPase activity and fatty acid composition in Pinus resinosa needles during cold acclimation and de-acclimation. Tree Physiol 26:783–790
Mattheis JP, Ketchie DO (1990) Changes in parameters of the plasmalemma ATPase during cold acclimation of apple (Malus domestica) tree bark tissues. Physiol Plant 78:616–622
Morandini P, Valera M, Albumi C, Bonza MC, Giacometti S, Murgia I, Soave C, De Michelis MI (2002) A novel interaction partner for the C-terminus of Arabidopsis thaliana plasma membrane H+-ATPase (AHA1 isoform): site and mechanism of action on H+-ATPase activity differ from those of 14-3-3 proteins. Plant J 31:487–497
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Olivari C, Albumi C, Pugliarello MC, De Michelis MI (2000) Phenylarsine oxide inhibits the fusicoccin-induced activation of plasma membrane H+-ATPase. Plant Physiol 122:463–470
Ottmann C, Marco S, Jaspert N, Marcon C, Schauer N, Weyand M, Vandermeeren C, Duby G, Boutry M, Wittinghofer A, Rigaud JL, Oecking C (2007) Structure of a 14-3-3 coordinated hexamer of the plant plasma membrane H+-ATPase by combining X-ray crystallography and electron cryomicroscopy. Mol Cell 25:427–440
País SM, González MA, Téllez-Iñón MT, Capiati DA (2009) Characterization of potato (Solanum tuberosum) and tomato (Solanum lycopersicum) protein phosphatases type 2A catalytic subunits and their involvement in stress responses. Planta 230:13–25
País SM, Muñíz García MN, Téllez-Iñón MT, Capiati DA (2010) Protein phosphatases type 2A mediate tuberization signaling in Solanum tuberosum L. leaves. Planta 232:37–49
Palmgren MG (2001) Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol 52:817–845
Papini R, De Michelis MI (1997) Changes in the level and activation state of the plasma membrane H+-ATPase during aging of red beet slices. Plant Physiol 114:857–862
Rensink WA, Lobst S, Hart A, Stegalkina S, Liu J, Buell CR (2005) Gene expression profiling of potato responses to cold, heat, and salt stress. Funct Integr Genomics 5:201–207
Rober-Kleber N, Albrechtová JT, Fleig S, Huck N, Michalke W, Wagner E, Speth V, Neuhaus G, Fischer-Iglesias C (2003) Plasma membrane H+-ATPase is involved in auxin-mediated cell elongation during wheat embryo development. Plant Physiol 13:1302–1312
Sahu BB, Shaw BP (2009) Salt-inducible isoform of plasma membrane H+-ATPase gene in rice remains constitutively expressed in natural halophyte, Suaeda maritima. J Plant Physiol 166:1077–1089
Sanchez-Serrano J, Scmidt R, Schell J, Willmitzer L (1986) Nucleotide sequence of proteinase inhibitor II encoding cDNA of potato (Solanum tuberosum) and its mode of expression. Mol Gen Genet 203:15–20
Schaller GE, DeWitt ND (1995) Analysis of H+-ATPase and other proteins of the Arabidopsis plasma membrane. Methods Cell Biol 50:129–148
Shi Y, An L, Zhang M, Huang C, Zhang H, Xu S (2008) Regulation of the plasma membrane during exposure to low temperatures in suspension-cultured cells from a cryophyte (Chorispora bungeana). Protoplasma 232:173–781
Sibole JV, Cabot C, Michalke W, Poschenrieder C, Barceló J (2005) Relationship between expression of the PM H+-ATPase, growth and ion partitioning in the leaves of salt-treated Medicago species. Planta 221:557–566
Sondergaard TE, Schulz A, Palmgren MG (2004) Energization of transport processes in plants. Roles of the plasma membrane H+-ATPase. Plant Physiol 136:2475–2482
Sveinsdóttir H, Yan F, Zhu Y, Peiter-Volk T, Schubert S (2009) Seed ageing-induced inhibition of germination and post-germination root growth is related to lower activity of plasma membrane H+-ATPase in maize roots. J Plant Physiol 166:128–135
Viotti C, Luoni L, Morandini P, De Michelis MI (2005) Characterization of the interaction between the plasma membrane H+-ATPase of Arabidopsis thaliana and a novel interactor (PPI1). FEBS J 272:5864–5871
Vitart V, Baxter I, Doerner P, Harper JF (2001) Evidence for a role in growth and salt resistance of a plasma membrane H+-ATPase in the root endodermis. Plant J 27:191–201
Wu J, Seliskar D (1998) Salinity adaptation of plasma membrane H+-ATPase in the salt marsh plant Spartina patens: ATP hydrolysis and enzyme kinetics. J Exp Bot 49:1005–1013
Yamagishi K, Mitsumori C, Takahashi K, Fujino K, Koda Y, Kikuta Y (1993) Jasmonic acid-inducible gene expression of a Kunitz-type proteinase inhibitor in potato tuber disks. Plant Mol Biol 21:539–541
Zhao R, Dielen V, Kinet JM, Boutry M (2000) Cosuppression of a plasma membrane H+-ATPase isoform impairs sucrose translocation, stomatal opening, plant growth, and male fertility. Plant Cell 12:535–546
Acknowledgment
We thank Salomé Vilchez (INGEBI-CONICET) for the generous gift of the TcPARP recombinant protein.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muñiz García, M.N., País, S.M., Téllez-Iñón, M.T. et al. Characterization of StPPI1, a proton pump interactor from Solanum tuberosum L. that is up-regulated during tuber development and by abiotic stress. Planta 233, 661–674 (2011). https://doi.org/10.1007/s00425-010-1329-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1329-0