Abstract
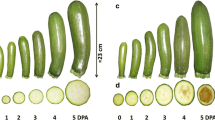
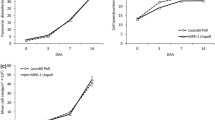
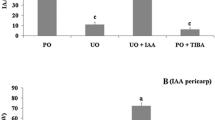
To analyze fruit set and early fruit (caryopsis) development in rice, we established an in vitro spikelet culture system. The ovary of cultured pollinated spikelets grew rapidly and developed into fruits with an embryo and endosperm. When unpollinated spikelets were cultured on a medium containing 2,4-dichlorophenoxyacetic acid, parthenocarpic fruits lacking an embryo and endosperm developed. The number and size of the cells in the pericarp of parthenocarpic fruits were almost identical to those of fruits induced by pollination, and degeneration of nucellus tissue was observed in both pollinated and parthenocarpic fruits. These results suggested that ovary growth was induced through increased auxin content in the spikelets. Quantitative measurement of indole-3-acetic acid (IAA) content in the spikelets indicated that the IAA level increased after pollination. Further analysis of IAA contents in the ovary and rachilla–pedicel of cultured spikelets suggested that fruit development is associated with IAA synthesis in the ovary following pollination/fertilization and subsequent transport of IAA from the ovary to the rachilla–pedicel. Partial or complete removal of the rachilla and/or pedicel prior to spikelet culture greatly inhibited fruit development. These results indicated that the rachilla and pedicel are essential for rice fruit development. AUX/IAA and ARF genes that might be involved in rice fruit development were identified through transcriptome analysis.







Similar content being viewed by others
Abbreviations
- IAA:
-
Indole-3-acetic acid
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- NPA:
-
1-N-naphthylphthalamic acid
- GA3 :
-
Gibberellin A3
References
Bajaj YPS (1966) Growth of Hyoscyamus niger ovaries in culture. Phyton 23:57–62
Beyer EM, Quebedeaux B (1974) Parthenocarpy in cucumber: mechanism of auxin transport inhibitors. J Am Soc Hortic Sci 99:385–390
Britten EJ (1950) Natural and induced parthenocarpy in maize and its relation to hormone production by developing seed. Am J Bot 37:345–352
Chopra RN (1962) Effect of some growth substances and calyx on fruit and seed development of Altheaea rosea Cav. In: Plant embryology—a symposium. Council of Scientific and Industrial Research, New Delhi, pp 170–181
de Jong M, Wolters-Arts M, Feron R, Mariani C, Vriezen WH (2009a) The Solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant J 57:160–170
de Jong M, Mariani C, Vriezen WH (2009b) The role of auxin and gibberellin in tomato fruit set. J Exp Bot 60:1523–1532
Dharmosiri N, Estelle M (2004) Auxin signaling and regulated protein degradation. Trends Plant Sci 9:302–308
Dorcey E, Urbez C, Blázquez MA, Carbonell J, Perez-Amador MA (2009) Fertilization-dependent auxin response in ovules triggers fruit development through the modulation of gibberellin metabolism in Arabidopsis. Plant J 58:318–332
Geldner N, Friml J, Stierhof YD, Jurgens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413:425–428
George WL, Scott JW, Splittstoesser WE (1984) Parthenocarpy in tomato. Hortic Rev 6:65–84
Goetz M, Vivian-Smith A, Johnson SD, Koltunow AM (2006) Auxin response factor8 is a negative regulator of fruit initiation in Arabidopsis. Plant Cell 18:1873–1886
Goetz M, Hooper LC, Johnson SD, Rodrigues CM, Vivian-Smith A, Koltunow M (2007) Expression of aberrant forms of auxin response factor 8 stimulates parthenocarpy in Arabidopsis and tomato. Plant Physiol 145:351–366
Goodwin PB (1978) Phytohormones and fruit growth. In: Letham DS, Goodwin PB, Higgins TJV (eds) Phytohormones and related compounds, a comprehensive treatise, vol 2. Elsevier, Amsterdam, pp 175–204
Granell A, Harris N, Pisabarro AG, Carbonell J (1992) Temporal and spatial expression of a thiolprotease gene during pea ovary senescence, and its regulation by gibberellin. Plant J 2:907–915
Guha S, Johri BM (1966) In vitro development of ovary and ovule of Allium cepa L. Phytomorphology 16:353–364
Gustafson FG (1936) Inducement of fruit development by growth promoting chemicals. Proc Natl Acad Sci USA 22:629–636
Gustafson FG (1939) The cause of natural parthenocarpy. Am J Bot 26:135–138
Higgins RK, Petolino JF (1988) In vitro pollination fertilization of maize: influence of explant factors on kernel development. Plant Cell Tissue Organ Cult 12:21–30
Hirano K, Aya K, Hobo T, Sakakibara H, Kojima M, Shim RA, Hasegawa Y, Ueguchi-Tanaka M, Matsuoka M (2008) Comprehensive transcriptome analysis of phytohormone biosynthesis and signaling genes in microspore/pollen and tapetum of rice. Plant Cell Physiol 49:1429–1450
Ishimaru T, Matsuda T, Ohsugi R, Yamagushi T (2003) Morphological development of rice caryopsis located at the different positions in a panicle from early to middle stage of grain filling. Funct Plant Biol 30:1139–1149
Ito I (1966) In vitro culture of ovary in orchid (1). Effects of sugar, peptone and coconut milk upon the growth ovary of Dendrobium nobile. Sci Rep Kyoto Prefect Univ Agric 18:38–50
Jain M, Kaur N, Garg R, Thankur JK, Tyagi AK, Khurana JP (2006) Structure and expression analysis of early auxin-responsive AUX/IAA gene family in rice (Oryza sativa). Funct Integr Genomics 6:47–59
Kano K (1962) In vitro culture of tomato ovaries. J Jpn Soc Hortic Sci 31:197–206 [In Japanese]
Keitt GW, Baker RA (1966) Auxin activity of substituted benzoic acids and their effect on polar auxin transport. Plant Physiol 41:1561–1569
Kim I, Okubo H, Fukuda K (1992) Endogenous levels of IAA in relation to parthenocapy in cucumber (Cucumis sativus L.). Sci Hortic 52:1–8
Krishnan S, Dayanandan P (2003) Structural and histochemical studies on grain-filling in the caryopsis of rice (Oryza sativa L.). J Biosci 28:455–469
LaCroix LJ, Naylor J, Larter EN (1962) Factors controlling embryo growth and development in barley (Hordeum vulgare L.). Can J Bot 40:1515–1523
LaRue CD (1942) The rooting flowers in sterile culture. Bull Torrey Botan Club 69:332–341
Leyser O (2006) Dynamic integration of auxin transport and signalling. Curr Biol 16:R424–R433
Maheshwari N, Lal M (1961) In vitro culture of ovaries of Iberis amara L. Phytomorphology 11:17–23
Mapelli S, Frova C, Torti G, Soressi GP (1978) Relationship between set, development and activities of growth regulators in tomato fruits. Plant Cell Physiol 19:1281–1288
Marshall DR, Molnár-Láng M, Ellison FW (1983) Effects of 2,4-D on parthenocarpy and cross-compatibility in wheat. Cereal Res Com 11:213–219
Mathias RJ, Boyd LA (1988) The growth in culture of detached ovaries of wheat (Triticum aestivum L. em. Thell). Plant Breed 100:143–146
Matsuo T, Hoshikawa K (1993) Science of the rice plant, vol 1: morphology. Food and Agriculture Policy Research Center, pp 293–412
Matzk F (1991) A novel approach to differentiated embryos in the absence of endosperm. Sex Plant Reprod 4:88–94
Mori Y, Nishimura T, Koshiba T (2005) Vigorous synthesis of indole-3-acetic acid in the apical very tip leads to a constant basipetal flow of the hormone in maize coleoptiles. Plant Sci 168:467–473
Ngo P, Ozga JA, Reinecke DM (2002) Specificity of auxin regulation of gibberellin 20-oxidase gene expression in pea pericarp. Plant Mol Biol 49:439–448
Nishimura T, Mori Y, Furukawa T, Kadota A, Koshiba T (2006) Red light causes a reduction in IAA levels at the apical tip by inhibiting de novo biosynthesis from tryptophan in maize coleoptiles. Planta 224:1427–1435
Nitsch JP (1950) Growth and morphogenesis of the strawberry as related to auxin. Am J Bot 37:211–215
Nitsch JP (1951) Growth and development in vitro of excised ovaries. Am J Bot 38:566–577
Nitsch JP (1952) Plant hormones in the development of fruits. Q Rev Biol 27:33–57
O’Neill SD, Nadeau JA (1997) Post-pollination flower development. Hortic Rev 19:1–58
Ozga JA, Reinecke DM (2003) Hormonal interactions in fruit development. J Plant Growth Regul 22:73–81
Ozga JA, Reinecke DM, Ayele BT, Ngo P, Nadeau C, Wickramarathna AD (2009) Developmental and hormonal regulation of gibberellin biosynthesis and catabolism in pea fruit. Plant Physiol 150:448–462
Pandolfini T, Molesini B, Spena A (2007) Molecular dissection of the role of auxin in fruit initiation. Trends Plant Sci 12:327–329
Rangan TS (1984) Culture of ovaries. Culture of ovules. In: Vasil IK (ed) Cell culture and somatic cell genetics of plants. Academic Press, New York, pp 221–231
Rédei G, Rédei G (1955) Rearing wheats from ovaries cultured in vitro. Acta Bot Acad Sci Hung 2:183–185
Sacher RC, Guha S (1962) In vitro growth of achenes of Ranunculus scleratus L. In: Plant embryology—a symposium. Council of Scientific and Industrial Research, New Delhi, India, pp 244–253
Serrani JC, Fos M, Atarés A, García-Martínez JL (2007) Effect of gibberellin and auxin on parthenocarpic fruit growth induction in the cv Micro-tom of tomato. J Plant Growth Regul 26:211–221
Serrani JC, Ruiz-Rivero O, Fos M, García-Martínez JL (2008) Auxin-induced fruit-set in tomato is mediated in part by gibberellins. Plant J 56:922–934
Shimizu M, Naito K (1968) Effects of gibberellin on the morphogenesis in rice plants: 10. Comparative effects of gibberellin and auxins on the induction of parthenocarpic caryopsis. Proc Crop Sci Soc Jpn 37:230–234 [in Japanese]
Sjut V, Bangerth B (1982) Induced parthenocarpy- a way of changing the levels of endogenous hormones in tomato fruits (Lycopersicon esculentum Mill.) 1. Extractable hormones. Plant Growth Regul 1:243–251
Talon M, Hedden P, Primo-Millo E (1990) Gibberellins in Citrus sinesis: a comparison between seeded and seedless varieties. J Plant Growth Regul 9:201–206
Talon M, Zacarias L, Primo-Millo E (1992) Gibberellins and parthenocarpic ability in developing ovaries of seedless mandarins. Plant Physiol 99:1575–1581
Vercher Y, Carbonell J (1991) Change in structure of ovary tissue and in the ultrastructure of mesocarp cells during ovary senescence or fruit development induced by plant growth substance in Pisum sativum. Physiol Plant 81:518–526
Vercher Y, Molowny A, Lopez C, García-Martínez JL, Carbonell J (1984) Structural changes in the owary of Pisum sativum L. induced by pollination and gibberellic acid. Plant Sci Lett 36:87–91
Vercher Y, Carrasco P, Carbonell J (1989) Biochemical and histochemical detection of endoproteolytic activities involved in ovary senescence or fruit development in Pisum sativum. Physiol Plant 76:405–411
Vivian-Smith A, Koltunow AM (1999) Genetic analysis of growth-regulator-induced parthenocarpy in Arabidopsis. Plant Physiol 121:437–451
Vivian-Smith A, Luo M, Chaudhury A, Koltunow AM (2001) Fruit development is actively restricted in the absence of fertilization in Arabidopsis. Development 128:2321–2331
Vriezen WH, Feron R, Maretto F, Keijman J, Mariani C (2008) Changes in tomato ovary transcriptome demonstrate complex hormonal regulation of fruit set. New Phytol 177:60–76
Wang H, Jones B, Li Z, Frasse P, Delalande C, Regad F, Chaabouni S, Latché A, Pech JC, Bouzayen M (2005) The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 17:2676–2692
Wang D, Pei K, Fu Y, Sun Z, Li S, Liu H, Tang K, Han B, Tao Y (2007) Genome-wide analysis of the auxin response factor (ARF) gene family in rice (Oryza sativa). Gene 394:13–24
Wittwer SH, Bukovac MJ, Sell HM, Weller LE (1957) Some effects of gibberellin on flowering and fruit setting. Plant Physiol 32:39–41
Woodward AW, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot 95:707–735
Acknowledgments
This work was supported by a Grant-in-Aid for JSPS fellows (Grant no. 2010366 to T. Uchiumi), and partly by “Program for Improving Graduate School Education” funds awarded to the Department of Biological Sciences, Graduate School of Tokyo Metropolitan University, by the Japanese Ministry of Education, Culture, Sports, Science and Technology. This work was also supported, in part, by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (no. 21112007 to T. Okamoto) and from the Japan Society for the Promotion of Science (no. 20570206 to T. Okamoto). We thank Dr. Y. Nagamura and his colleagues (National Institute of Agrobiological Sciences, Tsukuba, Japan) for assistance with the microarray experiment, and Dr. T. Koshiba and Dr. T. Nishimura (Tokyo Metropolitan University) for measurement of IAA content.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Uchiumi, T., Okamoto, T. Rice fruit development is associated with an increased IAA content in pollinated ovaries. Planta 232, 579–592 (2010). https://doi.org/10.1007/s00425-010-1197-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1197-7