Abstract
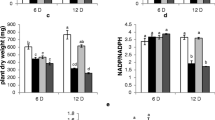
Metabolite profiles and activities of key enzymes in the metabolism of organic acids, nitrogen and amino acids were compared between chlorotic leaves and normal leaves of ‘Honeycrisp’ apple to understand how accumulation of non-structural carbohydrates affects the metabolism of organic acids, nitrogen and amino acids. Excessive accumulation of non-structural carbohydrates and much lower CO2 assimilation were found in chlorotic leaves than in normal leaves, confirming feedback inhibition of photosynthesis in chlorotic leaves. Dark respiration and activities of several key enzymes in glycolysis and tricarboxylic acid (TCA) cycle, ATP-phosphofructokinase, pyruvate kinase, citrate synthase, aconitase and isocitrate dehydrogenase were significantly higher in chlorotic leaves than in normal leaves. However, concentrations of most organic acids including phosphoenolpyruvate (PEP), pyruvate, oxaloacetate, 2-oxoglutarate, malate and fumarate, and activities of key enzymes involved in the anapleurotic pathway including PEP carboxylase, NAD-malate dehydrogenase and NAD-malic enzyme were significantly lower in chlorotic leaves than in normal leaves. Concentrations of soluble proteins and most free amino acids were significantly lower in chlorotic leaves than in normal leaves. Activities of key enzymes in nitrogen assimilation and amino acid synthesis, including nitrate reductase, glutamine synthetase, ferredoxin and NADH-dependent glutamate synthase, and glutamate pyruvate transaminase were significantly lower in chlorotic leaves than in normal leaves. It was concluded that, in response to excessive accumulation of non-structural carbohydrates, glycolysis and TCA cycle were up-regulated to “consume” the excess carbon available, whereas the anapleurotic pathway, nitrogen assimilation and amino acid synthesis were down-regulated to reduce the overall rate of amino acid and protein synthesis.






Similar content being viewed by others
Abbreviations
- ATP-PFK:
-
ATP-phosphofructokinase
- CS:
-
Citrate synthase
- DTT:
-
Dithiothreitol
- EDTA:
-
Ethylenediaminetetraacetic acid
- Fd-GOGAT:
-
Ferredoxin-dependent glutamate synthase
- F6P:
-
Fructose 6-phosphate
- G6P:
-
Glucose 6-phosphate
- GDH:
-
Glutamate dehydrogenase
- GOT:
-
Glutamate oxaloacetate transaminase
- GPT:
-
Glutamate pyruvate transaminase
- GS:
-
Glutamine synthetase
- HK:
-
Hexokinase
- ICDH:
-
Isocitrate dehydrogenase
- NADH-GOGAT:
-
NADH-dependent glutamate synthase
- NAD-MDH:
-
NAD-malate dehydrogenase
- NAD-ME:
-
NAD-malic enzyme
- NADP-ME:
-
NADP-malic enzyme
- NR:
-
Nitrate reductase
- OAA:
-
Oxaloacetate
- 2-OG:
-
2-Oxoglutarate
- PEP:
-
Phosphoenolpyruvate
- PEPC:
-
PEP carboxylase
- PK:
-
Pyruvate kinase
- PVPP:
-
Polyvinylpolypyrrolidone
- TCA:
-
Tricarboxylic acid
References
Andrews M (1986) The partitioning of nitrate assimilation between root and shoot of higher plants. Plant Cell Environ 9:511–519
Bergmeyer H, Grassl M, Walter H (1983) In: Bergmeyer HU (ed) Methods in enzymatic analysis, vol 2. VCH, Weinheim, pp 273–274
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Bryan JK (1980) Synthesis of the aspartate family and branched-chain amino acids. In: Miflin BJ (ed) The biochemistry of plants: a comprehensive treatise, vol 5. Academic Press, New York, pp 403–452
Cave G, Tolley LC, Strain BR (1981) Effect of carbon dioxide enrichment on chlorophyll content, starch content and starch grain structure in Trifolium subterraneum leaves. Physiol Plant 51:171–174
Chen L, Cheng L (2004) CO2 assimilation, carbohydrate metabolism, xanthophyll cycle, and antioxidant system of ‘Honeycrisp’ apple leaves with zonal chlorosis. J Amer Soc Hort Sci 129:729–737
Chen LS, Lin Q, Nose A (2002) A comparative study on diurnal changes in metabolite levels in the leaves of three crassulacean acid metabolism (CAM) species, Ananas somosus, Kalanchoë daigremontiana and K. pinnata. J Exp Bot 53:341–350
Chen F, Liu X, Chen L (2009) Developmental changes in pulp organic acid concentration and activities of two loquat (Eriobotrya japonica Lindl) cultivars differing in fruit acidity. Food Chem 114:657–664
Cohen SA, Michaud DP (1993) Synthesis of a fluorescent derivatizing reagent, 6-amino-quinolyl-N-hydroxysuccinimidyl carbamate and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal Biochem 211:279–287
Cui L, Cao R, Li J, Zhang L, Wang J (2006) High temperature effects on ammonium assimilation in leaves of two Festuca arundinacea cultivars with different heat susceptibility. Plant Growth Regul 49:127–136
Du YC, Nose A, Wasano K, Uchida Y (1998) Responses to water stress of enzyme activities and metabolite levels in relation to sucrose and starch synthesis, the Calvin cycle and the C4 pathway in sugarcane (Saccharum sp.) leaves. Aust J Plant Physiol 25:253–260
Emmerlich V, Linka N, Reinhold T, Hurth MA, Traub M, Martinoia E, Neuhaus HE (2003) The plant homolog to the human sodium/dicarboxylic cotransporter is the vacuolar malate carrier. Proc Natl Acad Sci USA 100:11122–11126
Geiger M, Walch-Liu P, Engels C, Harnecker J, Shulze ED, Ludewig F, Sonnewald U, Scheible WR, Stittt M (1998) Enhanced carbon dioxide leads to a modified diurnal rhythm of nitrate reductase activity in older plants, and a large stimulation of nitrate reductase activity and higher levels of amino acids in young tobacco plants. Plant Cell Environ 21:253–268
Goldschmidt EE, Huber SC (1992) Regulation of photosynthesis by end-product accumulation in leaves of plants storing starch, sucrose, and hexose sugars. Plant Physiol 99:1443–1448
Hageman RH, Reed AJ, Femmer RA, Sherrard JH, Dalling MJ (1980) Some new aspects of the in vivo assay for nitrate reductase in wheat (Triticum aestivum L.) leaves, 1: re-evaluation of nitrate pool sizes. Plant Physiol 65:27–32
Häusler RE, Fischer KL, Flügge UI (2000) Determination of low-abundant metabolites in plant extracts by NAD(P)H fluorescence with a microtiter plate reader. Anal Biochem 281:1–8
Häusler RE, Thomas R, Li J, Lipka V, Fischer KL, Schuber S, Kreuzaler F, Hirsch H (2001) Single and double overexpression of C4-cycle genes had differential effects on the pattern of endogenous enzymes, attenuation of photorespiration and on contents of UV protectants in transgenic potato and tobacco plants. J Exp Bot 52:1785–1803
Hayashi F, Ichino T, Osanai M, Wada K (2000) Oscillation and regulation of proline content by P5CS and ProDH gene expressions in the light/dark cycles in Arabidopsis thaliana L. Plant Cell Physiol 41:1096–1101
Hill SA (1997) Carbon metabolism in mitochondria. In: Dennis DT, Turpin DH, Lefebvre DD, Layzell DB (eds) Plant metabolism. Addison Welsey Longman, London, pp 181–199
Hurth MA, Suh SJ, Kretzschmar T, Geis T, Bregante M, Gambale F, Martinoia E, Neuhaus HE (2005) Impaired pH homeostasis in Arabidopsis lacking the vacuolar dicarboxylate transporter and analysis of carboxylic acid transport across the tonoplast. Plant Physiol 137:901–910
Kar M, Feierabend J (1984) Changes in the activities of enzymes involved in amino acid metabolism during senescence of detached wheat leaves. Physiol Plant 62:39–44
Keutgen N, Chen K, Lenz F (1997) Responses of strawberry leaf photosynthesis, chlorophyll fluorescence and macronutrient contents to elevated CO2. J Plant Physiol 150:395–400
Krapp A, Stitt M (1995) An evaluation of direct and indirect mechanisms for the “sink-regulation” of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 195:313–323
Krapp A, Hofmann B, Schäfer C, Stitt M (1993) Regulation of the expression of rbcS and other photosynthetic genes by carbohydrates: a mechanism for the ‘sink regulation’ of photosynthesis? Plant J 3:817–826
Kumar RG, Shanh K, Dubey RS (2000) Salinity induced behavioral changes in malate dehydrogenase and glutamate dehydrogenase activities in rice seedlings of differing salt tolerance. Plant Sci 156:23–24
Labboun S, Tercé-Laforgue T, Roscher A, Bedu M, Restivo FM, Velanis CN, Skopelitis DS, Moshou PN, Roubelakis-Angelakis KA, Suzuku A, Hirel B (2009) Resolving the role of plant glutamate dehydrogenase, I: in vivo real time nuclear magnetic resonance spectroscopy experiments. Plant Cell Physiol 50:1761–1773
Lea PJ, Robinson SA, Stewart GR (1990) The enzymology and metabolism of glutamine, glutamate, and asparagine. In: Miflin BJ, Lea PJ (eds) The biochemistry of plants: intermediary nitrogen metabolism. Academic Press, New York, pp 121–159
Leakey ADB, Xu F, Gillespie KM, McGrath JM, Ainsworth EA, Ort DR (2009) Genomic basis for stimulated respiration by plants growing under elevated carbon dioxide. Proc Natl Acad Sci USA 106:3597–3602
Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR (2006) Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc 1:387–396
Lutts S, Majerus V, Kinet JM (1999) NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiol Plant 105:450–458
Melzer E, O’Leary M (1987) Anapleurotic CO2 fixation by phosphoenolpyruvate carboxylase in C3 plants. Plant Physiol 84:58–60
Morcuende R, Perez P, Martinez-carrasco R, Molino IM, Puente LS (1996) Long- and short-term effects of decreased sink demand on carbohydrate levels and photosynthesis in wheat leaves. Plant Cell Environ 19:1203–1209
Noguchi K, Yoshida K (2008) Interaction between photosynthesis and respiration in illuminated leaves. Mitochondrion 8:87–99
Pistelli G, Marigo G, Ball E, Lüttge U (1987) Day–night changes in the levels of adenine nucleotides, phosphoenolpyruvate and inorganic pyrophosphate in leaves of plants having Crassulacean acid metabolism. Planta 172:479–486
Ramajulu S, Vlerajaniyulu K, Sudhakar C (1994) Short-term shifts in nitrogen metabolism in mulberry Morus alba under salt shock. Phytochemistry 35:991–995
Reidel RJ, Rennie EA, Amiard V, Cheng L, Turgeon R (2009) Phloem loading strategies in three plant species that transport sugar alcohols. Plant Physiol 149:1601–1608
Rentsch D, Martinola E (1991) Citrate transport into barley mesophyll vacuoles–comparison with malate-uptake activity. Planta 184:532–537
Robinson TL, Watkins CB (2003) Cropload of ‘Honeycrisp’ affects not only fruit size but many quality attributes. New York Fruit Q 11(3):7–10
Rogers A, Gibon Y, Stitt M, Morgan PB, Bernacchi CJ, Ort DR, Long SP (2006) Increased C availability at elevated carbon dioxide concentration improves N assimilation in a legume. Plant Cell Environ 29:1651–1658
Rosenberger DA, Schupp JR, Watkins CB, Iungerman K, Hoying S, Straub D, Cheng L (2001) ‘Honeycrisp’: a darling new apple variety or just another problem child? New York Fruit Q 9(3):9–13
Sawada S, Sakamoto T, Sato M, Kasai M, Usuda H (2002) Photosynthesis with single-rooted Amaranthus leaves. II. Regulation of ribulose-1, 5-bisphosphate carboxylase, phosphoenolpyruvate carboxylase, NAD-malic enzyme and NAD-malate dehydrogenase and coordination between PCR and C4 photosynthetic metabolism in response to changes in the source–sink balance. Plant Cell Physiol 43:1293–1301
Schaffer AA, Liu KC, Goldschmidt EE, Boyer CD, Goren R (1986) Citrus leaf chlorosis induced by sink removal: starch, nitrogen, and chloroplast ultrastructure. J Plant Physiol 124:111–121
Scheible WR, Gonzales-Fontes A, Lauerer M, Müller-Röber B, Caboche M, Stitt M (1997) Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell 9:783–798
Schupp JR (2003) Effects of chemical thinners on fruit set, yield, fruit size, and fruit quality of ‘Honeycrisp’ apple. New York Fruit Q 11(3):3–5
Schupp JR, Ferree DC, Warrington IJ (1992) Interaction of root pruning and deblossoming on growth, development and yield of ‘Golden Delicious’ apple. J Hortic Sci 67:465–480
Sicher RC (1998) Yellowing and photosynthetic decline of barley primary leaves in response to atmospheric CO2 enrichment. Physiol Plant 103:193–200
Sicher RC (2008) Effects of CO2 enrichment on soluble amino acids and organic acids in barley primary leaves as a function of age, photoperiod and chlorosis. Plant Sci 174:576–582
Singh DP, Verma K (1996) Differential regulation of nitrogen metabolism in the wild type and the high-light-tolerant mutant cells of Anacysis nidulans. Curr Microbiol 32:272–278
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Stitt M, von Schaewen A, Willmitzer L (1991) ‘Sink’ regulation of photosynthetic metabolism in transgenic tobacco plants expressing yeast invertase of glycolytic enzymes. Planta 183:40–50
Thomas H (1978) Enzymes of nitrogen mobilization in detached leaves of Lolium temulentum during senescence. Planta 142:161–169
Trethewey RN, Geigenberger P, Riedel K, Hajirezaei MR, Sonnewald U, Stitt M, Riesmeier JW, Willmitzer L (1998) Combined expression of glucokinase and invertase in potato tubers leads to a dramatic reduction in starch accumulation and a stimulation of glycolysis. Plant J 15:109–118
Trethewey RN, Riesmeier JW, Willmitzer L, Stitt M, Geigenberger P (1999) Tuber-specific expression of a yeast invertase and a bacterial glucokinase in potato leads to an activation of sucrose phosphate synthase and the creation of a futile cycle. Planta 208:227–238
Wahlefeld AW (1974) Oxalacetate: UV spectrophotometric determination. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 3. Academic Press, New York, pp 1604–1608
Wang ZQ, Yuan YZ, Ou YQ, Lin QH, Zhang CF (2007) Glutamine synthetase and glutamate dehydrogenase contribute differentially to proline accumulation in leaves of wheat (Triticum aestivum) seedlings exposed to different salinity. J Plant Physiol 164:695–701
Wullschleger SD, Borby RJ, Hendrix DL (1992) Carbon exchange rates, chlorophyll content, and carbohydrate status of two forest tree species exposed to carbon dioxide enrichment. Tree Physiol 10:21–31
Zhou R, Quebedeaux B (2003) Changes in photosynthesis and carbohydrate metabolism in mature apple leaves in response to whole plant source–sink manipulation. J Am Soc Hort Sci 128:113–119
Acknowledgments
The Agilent GC/MS system used in this work was generously donated by Dr. David Zimerman, Cornell Pomology Ph.D. 1954. The authors would like to thank Drs. Alisdair Fernie and Maria Ines at Max Planck Institute of Molecular Plant Physiology in Germany for their valuable advice and help on metabolite profiling using GC/MS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Ma, F. & Cheng, L. Metabolism of organic acids, nitrogen and amino acids in chlorotic leaves of ‘Honeycrisp’ apple (Malus domestica Borkh) with excessive accumulation of carbohydrates. Planta 232, 511–522 (2010). https://doi.org/10.1007/s00425-010-1194-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1194-x