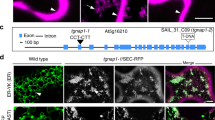
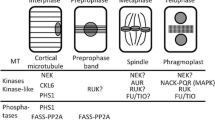
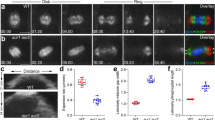
Abstract
The organization of plant microtubule arrays is thought to be regulated by phosphorylation and other signaling cascades, but the molecular components involved are largely unknown. We have previously found that a dominant missense mutation (phs1-1) in a putative kinase-docking motif of an Arabidopsis PHS1 phosphatase, which belongs to the mitogen-activated protein kinase phosphatase (MKP) family, compromises the stability of cortical microtubules. We here report that suppressor screening of phs1-1 recovered several intragenic recessive mutations in PHS1. In contrast to our previous report, null alleles of PHS1 were almost indistinguishable from the wild type in morphology, but their roots skewed to the abnormal direction when grew in the presence of low doses of a microtubule-destabilizing drug. PHS1 is mainly expressed in elongating cells, where the protein was distributed in the cytoplasm, predominantly in a microsomal fraction. Recruitment of green fluorescent protein-tagged PHS1 in endomembrane aggregates after treatment with brefeldin A or in an endomembrane-organization mutant suggests that an association with endomembranes retains PHS1 in the cytoplasm. A nuclear export signal identified in the C-terminal tail also contributes to the robust cytoplasmic retention of PHS1.






Similar content being viewed by others
Abbreviations
- BFA:
-
Brefeldin A
- GFP:
-
Green fluorescent protein
- GUS:
-
β-glucuronidase
- LMB:
-
Leptomycin B
- MKP:
-
MAP kinase phosphatase
- MPK:
-
Mitogen-activated protein kinase
- NES:
-
Nuclear export signal
- NLS:
-
Nuclear localization signal
References
Alonso A, Sasin J, Bottini N et al (2004) Protein tyrosine phosphatases in the human genome. Cell 117:699–711
Bartels S, Anderson JC, Besteiro MAG, Carreri A, Hirt H, Buchala A, Métraux J-P, Peck SC, Ulm R (2009) MAP KINASE PHOSPHATASE1 and PROTEIN TYROSINE PHOSPHATASE1 are repressors of salicylic acid synthesis and SNC1-mediated responses in Arabidopsis. Plant Cell 21:2884–2897
Bogerd HP, Fridell RA, Benson RE, Hua J, Cullen BR (1996) Protein sequence requirements for function of the human T-cell leukemia virus type 1 Rex nuclear export signal delineated by a novel in vivo randomization-selection assay. Mol Cell Biol 16:4207–4214
Camilleri C, Azimzadeh J, Pastuglia M, Bellini C, Grandjean O, Bouchez D (2002) The Arabidopsis TONNEAU2 gene encodes a putative novel protein phosphatase 2A regulatory subunit essential for the control of the cortical cytoskeleton. Plant Cell 14:833–845
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Dickinson RJ, Keyse SM (2006) Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Sci 119:4607–4615
Dong X, Biswas A, Sűel KE, Jackson LK, Martinez R, Gu H, Chook YM (2009) Structural basis for leucine-rich nuclear export signal recognition by CRM1. Nature 458:1136–1141
Ehrhardt DW (2008) Straighten up and fly right—microtubule dynamics and organization of non-centrosomal arrays in higher plants. Curr Opin Cell Biol 20:107–116
Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Höfte H (1997) Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol 114:295–305
Gupta R, Huang Y, Luan S (1998) Identification of a dual-specificity protein phosphatase that inactivates a MAP kinase from Arabidopsis. Plant J 16:581–589
Haasen D, Kőhler C, Neuhaus G, Merkle T (1999) Nuclear export of proteins in plants: AtXPO1 is the export receptor for leucine-rich nuclear signals in Arabidopsis thaliana. Plant J 20:695–705
Hussey P, Hashimoto T (2008) The cytoskeleton and signal transduction: role and regulation of plant actin- and microtubule-binding proteins. Ann Plant Rev 33:244–272
Ishida T, Hashimoto T (2007) An Arabidopsis thaliana tubulin mutant with conditional root-skewing phenotype. J Plant Res 120:635–640
Ishida T, Thitamadee S, Hashimoto T (2007) Twisted growth and organization of cortical microtubules. J Plant Res 120:61–70
Kerk D, Bulgrien J, Smith DW, Barsam B, Veretnik S, Bribskov M (2002) The complement of protein phosphatase catalytic subunits encoded in the genome of Arabidopsis. Plant Physiol 129:908–925
Kosugi S, Hasebe M, Tomita M, Yanagawa H (2008) Nuclear export signal consensus sequences defined using a localization-based yeast selection system. Traffic 9:2053–2062
Lee JS, Ellis BE (2007) Arabidopsis MAPK phosphatase 2 (MKP2) positively regulates oxidative stress tolerance and inactivates the MPK3 and MPK6 MAPKs. J Biol Chem 282:25020–25029
Lee JS, Wang S, Sritubtim S, Chen J, Ellis BE (2009) Arabidopsis mitogen-activated protein kinase MPK12 interacts with the MAPK phosphatase IBR5 and regulates auxin signaling. Plant J 57:975–985
Liu Y, Shepherd EG, Nelin LD (2007) MAPK phosphatases—regulating the immune response. Nature Rev Immunol 7:202–212
Meskiene I, Baudouin E, Schweighofer A, Liwosz A, Jonak C, Rodriguez PL, Jelinek H, Hirt H (2003) Stress-induced protein phosphatase 2C is a negative regulator of mitogen-activated protein kinase. J Biol Chem 278:18945–18952
Monroe-Augustus M, Zolman BK, Bartel B (2003) IBR5, a dual-specificity phosphatase-like protein modulating auxin and abscisic acid responsiveness in Arabidopsis. Plant Cell 15:2979–2991
Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T (2007) Development of series of Gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng 104:34–41
Nakajima K, Furutani I, Tachimoto H, Matsubara H, Hashimoto T (2004) SPIRAL1 encodes a plant-specific microtubule-localized protein required for directional control of rapidly expanding Arabidopsis cells. Plant Cell 16:1178–1190
Naoi K, Hashimoto T (2004) A semi-dominant mutation in an Arabidopsis mitogen-activated protein kinase phosphatase-like gene compromises cortical microtubule organization. Plant Cell 16:1841–1853
Pemberton LF, Paschal BM (2005) Mechanisms of receptor-mediated nuclear import and export. Traffic 6:187–198
Quettier A, Bertrand C, Habricot V, Miginiac E, Agnes C, Jeannette E, Maldiney R (2006) The phs1-3 mutation in a putative dual-specificity protein tyrosine phosphatase gene provides hypersensitive responses to abscisic acid in Arabidopsis thaliana. Plant J 47:711–719
Sasabe M, Machida Y (2006) MAP65: a bridge linking a MAP kinase to microtubule turnover. Curr Opin Plant Biol 9:563–570
Schweighofer A, Kazanaviciute V, Scheiki E et al (2007) The PP2C-type phosphatase AP2C1, which negatively regulates MPK4 and MPK6, modulates innate immunity, jasmonic acid, and ethylene levels in Arabidopsis. Plant Cell 19:2213–2224
Shoji T, Suzuki K, Abe T, Kaneko Y, Shi H, Zhu J, Rus A, Hasegawa PM, Hashimoto T (2006) Salt stress affects cortical microtubule organization and helical growth in Arabidopsis. Plant Cell Physiol 47:1158–1168
Stewart AE, Dowd S, Keyse SM, McDonald NQ (1999) Crystal structure of the MAPK phosphatase Pyst1 catalytic domain and implications for regulated activation. Nat Struct Biol 6:174–181
Tamura K, Shimada T, Kondo M, Nishimura M, Hara-Nishimura I (2007) KATAMARI1/MURUS3 is a novel Golgi membrane protein that is required for endomembrane organization in Arabidopsis. Plant Cell 17:1764–1776
Ulm R, Ichimura K, Mizoguchi T, Peck SC, Zhu T, Wang X, Shinozaki K, Paszkowski J (2002) Distinct regulation of salinity and genotoxic stress responses by Arabidopsis MAP kinase phosphatase 1. EMBO J 21:6483–6493
Walia A, Lee JS, Wasteneys G, Ellis B (2009) Arabidopsis mitogen-activated protein kinase MPK18 mediates cortical microtubule functions in plant cells. Plant J 59:565–575
Wasteneys GO (2002) Microtubule organization in the green kingdom: chaos or self-order? J Cell Sci 115:1345–1354
Wasteneys GO, Ambrose JC (2009) Spatial organization of plant cortical microtubules: close encounters of the 2D kind. Trends Cell Biol 19:62–71
Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2:1565–1572
Acknowledgments
We thank Thomas Merkle (University of Bielefeld) for pGFP-NLS-CHS-NESRev, Tsuyoshi Nakagawa (Shimane University) for pGWB plasmids, Roger Tsien (University of California at San Diego) for the mCherry construct, Ikuko Hara-Nishimura (Kyoto University) for kam1 mutant seeds, Masayoshi Maeshima (Nagoya University) for anti-v-ATPase antibody, and Minoru Yoshida (RIKEN) for leptomycin B. We are also grateful to Kuniko Naoi for her initial screening of suppressor mutants of phs1-1. The Arabidopsis Biological Resource Center is acknowledged for providing T-DNA and transposon insertion alleles of PHS1. J. Pytela was a recipient of a MEXT scholarship for graduate students. This research was supported by Grant-in-Aid for Scientific Research (B) 20370023, Grant-in-Aid for Scientific Research on Priority Areas 20064023 and the Global CEO Program of NAIST (Frontier Biosciences: strategies for survival and adaptation in a changing global environment), from MEXT, Japan, to T. Hashimoto.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pytela, J., Kato, T. & Hashimoto, T. Mitogen-activated protein kinase phosphatase PHS1 is retained in the cytoplasm by nuclear extrusion signal-dependent and independent mechanisms. Planta 231, 1311–1322 (2010). https://doi.org/10.1007/s00425-010-1135-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1135-8