Abstract
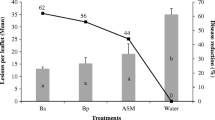
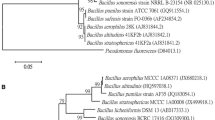
Root inoculation of tomato (Solanum lycopersicum) plants with a Bacillus subtilis strain BEB-DN (BsDN) isolated from the rhizosphere of cultivated potato plants was able to promote growth and to generate an induced systemic resistance (ISR) response against virus-free Bemisia tabaci. Growth promotion was evident 3 weeks after inoculation. No changes in oviposition density, preference and nymphal number in the early stages of B. tabaci development were observed between BsDN-treated plants and control plants inoculated with a non-growth promoting Bs strain (PY-79), growth medium or water. However, a long-term ISR response was manifested by a significantly reduced number of B. tabaci pupae developing into adults in BsDN-treated plants. The observed resistance response appeared to be a combination of jasmonic acid (JA) dependent and JA-independent responses, since the BsDN-related retardation effect on B. tabaci development was still effective in the highly susceptible spr2 tomato mutants with an impaired capacity for JA biosynthesis. A screening of 244 genes, 169 of which were previously obtained from subtractive-suppressive-hybridization libraries generated from B. tabaci-infested plants suggested that the BsDN JA-dependent ISR depended on an anti-nutritive effect produced by the simultaneous expression of genes coding principally for proteases and proteinase inhibitors, whereas the JA-independent ISR observed in the spr2 background curiously involved the up-regulation of several photosynthetic genes, key components of the phenyl-propanoid and terpenoid biosynthetic pathways and of the Hsp90 chaperonin, which probably mediated pest resistance response(s), in addition to the down-regulation of pathogenesis and hypersensitive response genes.







Similar content being viewed by others
Abbreviations
- Cfu:
-
Colony forming units
- JA:
-
Jasmonic acid
- MeSA:
-
Methyl salicylate
- SA:
-
Salicylic acid
- SAR:
-
Systemic acquired resistance
- ISR:
-
Induced systemic response
- spr2 :
-
Suppressor of prosystemin-mediated responses2
References
Achuo EA, Prinsen E, Höfte M (2006) Influence of drought, salt stress and abscisic acid on the resistance of tomato to Botrytis cinerea and Oidium neolycopersici. Plant Pathol 55:178–186
Ahn IP, Lee SW, Suh SC (2007) Rhizobacteria-induced priming in Arabidopsis is dependent on ethylene, jasmonic acid, and NPR1. Mol Plant Microbe Interact 20:759–768
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Attaran E, Zeier TE, Griebel T, Zeier J (2009) Methyl salicylate production and jasmonate signaling are not essential for systemic acquired resistance in Arabidopsis. Plant Cell 21:954–971
Bellés JM, López-Gresa MP, Fayos J, Pallás V, Rodrigo I, Conejero V (2008) Induction of cinnamate 4-hydroxylase and phenylpropanoids in virus-infected cucumber and melon plants. Plant Sci 174:524–533
Bhattarai KK, Li Q, Liu Y, Dinesh-Kumar SP, Kaloshian I (2007) The Mi-1-mediated pest resistance requires Hsp90 and Sgt11. Plant Physiol 144:312–323
Brown JK, Czosnek H (2002) Whitefly transmission of plant viruses. Adv Bot Res 36:65–92
Cartieaux F, Contesto C, Gallou A, Desbrosses G, Kopka J, Taconnat L, Renou JP, Touraine B (2008) Simultaneous interaction of Arabidopsis thaliana with Bradyrhizobium Sp. Strain ORS278 and Pseudomonas syringae pv. tomato DC3000 leads to complex transcriptome changes. Mol Plant Microbe Interact 21:244–259
Chacón O, Hernández I, Portieles R, López Y, Pujol M, Borrás-Hidalgo O (2009) Identification of defense-related genes in tobacco responding to black shank disease. Plant Sci 177:175–180
Chen M-S (2008) Inducible direct plant defense against insect herbivores: a review. Insect Sci 15:101–114
Chen H, Wilkerson CG, Kuchar JA, Phinney BS, Howe GA (2005) Jasmonate-inducible plant enzymes degrade essential amino acids in the herbivore midgut. Proc Natl Acad Sci USA 102:18771–18772
Cock MJN (1986) Bemisia tabaci—a literature survey on the cotton whitefly with an annotated bibliography. FAO/CAB International Institute of Biological Control, Ascot, UK, pp 21
Conrath U, Pieterse CMJ, Mauch-Mani B (2002) Priming in plant–pathogen interactions. Trends Plant Sci 7:210–216
Conrath U, Beckers GJM, Flors V, García-Agustín P, Jakab G, Mauch F, Newman M-A, Pieterse CMJ, Poinssot B, Pozo MJ, Pugin A, Schaffrath U, Ton J, Wendehenne W, Zimmerli L, Mauch-Mani B (2006) Priming: getting ready for battle. Mol Plant Microbe Interact 19:1062–1071
Czosnek H, Laterrot H (1997) A worldwide survey of tomato yellow leaf curl viruses. Arch Virol 142:1391–1406
de la Fuente van Bentem S, Vossen JH, de Vries KJ, van Wees S, Tameling WIL, Dekker HL, de Koster CG, Haring MA, Takken FLW, Cornelissen BJC (2005) Heat shock protein 90 and its co-chaperone protein phosphatase 5 interact with distinct regions of the tomato I-2 disease resistance protein. Plant J 43:284–298
Doelling JH, Walker JM, Friedman EM, Thompson AR, Vierstra RD (2002) The APG8/12-activating enzyme APG7 is required for proper nutrient recycling and senescence in Arabidopsis thaliana. J Biol Chem 277:33105–33114
Douglas AE (2006) Phloem-sap feeding by animals: problems and solutions. J Exp Bot 57:747–754
Duijff BJ, Gianinazzi-Pearson V, Lemanceau P (1997) Involvement of the outer membrane lipopolysaccharides in the endophytic colonization of tomato roots by biocontrol Pseudomonas fluorescens strain WCS417r. New Phytol 135:325–334
Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42:185–209
Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95:14863–14868
Estrada-Hernández MG, Valenzuela-Soto JH, Ibarra-Laclette E, Délano-Frier JP (2009) Differential gene expression in whitefly Bemisia tabaci-infested tomato (Solanum lycopersicum) plants at progressing developmental stages of the insect’s life cycle. Physiol Plant 137:44–60
Felton GW (2005) Indigestion is a plant’s best defense. Proc Natl Acad Sci USA 102:18771–18772
Frost CJ, Mescher MC, Carlson JE, De Moraes CM (2008) Plant defense priming against herbivores: getting ready for a different battle. Plant Physiol 146:818–824
Germain H, Chevalier E, Caron S, Matton DP (2005) Characterization of five RALF-like genes from Solanum chacoense provides support for a developmental role in plants. Planta 220:447–454
Gopalan S, Wei W, He SY (1996) hrp gene-dependent induction of hin 1: a plant gene activated rapidly by both harpins and the avrPto gene-mediated signal. Plant J 10:591–600
Hanafi A, Traoré M, Schnitzler WH, Woitke M (2007) Induced resistance of tomato to whiteflies and Phytium with the PGPR Bacillus subtilis in a soilless crop grown under greenhouse conditions. In: Hanafi A, Schnitzler WH (eds) Proceedings of VIIIth IS on protected cultivation in mild winter climates. Acta horticulturae, vol 747, pp 315–322
Herbette S, Lenne C, Leblanc N, Julien J-L, Drevet JR, Roeckel-Drevet P (2002) Two GPX-like proteins from Lycopersicon esculentum and Helianthus annuus are antioxidant enzymes with phospholipid hydroperoxide glutathione peroxidase and thioredoxin peroxidase activities. Eur J Biochem 269:2414–2420
Hermosa MR, Turra D, Foglianoc V, Montea E, Lorito M (2006) Identification and characterization of potato protease inhibitors able to inhibit pathogenicity and growth of Botrytis cinerea. Physiol Mol Plant Pathol 68:138–148
Hoelmer KA, Osborne LS, Yokomi RK (1991) Foliage disorders in Florida associated with feeding by sweetpotato whitefly, Bemisia tabaci. Florida Entomol 74:162–166
Howe GA (2004) Jasmonates as signals in the wound response. J Plant Growth Regul 23:223–237
Howe GA, Ryan CA (1999) Suppressors of systemin signaling identify genes in the tomato wound response pathway. Genetics 153:1411–1421
Jiménez-Delgadillo MR (1999) Evaluación y caracterización fisiológica de rizobacterias empleadas como posibles agentes de biocontrol. MSc Dissertation. Cinvestav I.P.N. Unidad Irapuato, México
Jones DR (2003) Plant viruses transmitted by whiteflies. Eur J Plant Pathol 109:195–219
Jordá L, Conejero V, Vera P (2000) Characterization of two differentially regulated genes (P69E and P69F) encoding new members of the subtilisin-like protease clan from tomato plants. Plant Physiol 122:67–74
Kazan K, Manners JM (2008) Jasmonate signaling: toward an integrated view. Plant Physiol 146:1459–1468
Kempema LA, Cui X, Holzer FM, Walling LL (2007) Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs. Plant Physiol 143:849–865
Kloepper JW, Tuzun S, Ku J (1992) Proposed definitions related to induced disease resistance. Biocontrol Sci Technol 2:349–351
Kloepper JW, Ryu CM, Zhang SA (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94:1259–1266
Koornneef A, Pieterse CMJ (2008) Cross-talk in defense signaling. Plant Physiol 146:839–844
Koornneef A, Leon-Reyes A, Ritsema T, Verhage A, Den Otter FC, Van Loon LC, Pieterse CMJ (2008) Kinetics of salicylate-mediated suppression of jasmonate signaling reveal a role for redox modulation. Plant Physiol 147:1358–1368
Leeman M, Van Pelt JA, Hendrickx MJ, Scheffer RJ, Bakker PAHM, Schippers B (1995) Biocontrol of fusarium wilt of radish in commercial greenhouse trials by seed treatment with Pseudomonas fluorescens WCS374. Phytopathology 85:1301–1305
Li C, Liu G, Xu C, Lee GI, Bauer P, Ling H-Q, Ganal MW, Howe GA (2003) The tomato suppressor of prosystemin-mediated responses2 gene encodes a fatty acid desaturase required for the biosynthesis of jasmonic acid and the production of a systemic wound signal for defense gene expression. Plant Cell 15:1646–1661
Liu Y, Burch-Smith T, Schiff M, Feng S, Dinesh-Kumar SP (2004) Molecular chaperone Hsp90 associates with resistance protein n and its signaling proteins SGT1 and Rar1 to modulate an innate immune response in plants. J Biol Chem 279:2101–2108
Mayer RT, McCollum TG, McDonald RE, Polston JE, Doostdar H (1995) Bemisia feeding induces pathogenesis-related proteins in tomato. In: Gerling D, Mayer RT (eds) Bemisia: 1995. Taxonomy, biology, damage, control and management, Andover Intercept Ltd., pp 179–188
Maynard DN, Cantliffe DJ (1989) Squash silverleaf and irregular ripening: new vegetable disorders in Florida. Fla Coop Ext Ser IFAS VC-37, pp 4
McAuslane HJ, Cheng J, Carle RB, Schmalstig J (2004) Influence of Bemisia argentifolii (Homoptera: Aleyrodidae) infestation and squash silver leaf disorder on zucchini seedling growth. J Econ Entomol 97:1096–1105
McKenzie CL, Sisisterra XH, Powell CA, Bausher M, Albano JP, Shatters RG Jr (2005) Deciphering changes in plant physiological response to whitefly feeding using microarray technology. In: Momol MT, Ji P, Jones JB (eds) Proceedings of the first international symposium on tomato diseases. Acta Horticulturae vol 695, pp 347–351
Merkouropoulos G, Barnett DC, Shirsat AH (1999) The Arabidopsis extensin gene is developmentally regulated, is induced by wounding, methyl jasmonate abscisic and salicylic acid, and codes for a protein with unusual motifs. Planta 208:212–219
Murphy JF, Zehnder GW, Shuster DJ, Sikora EJ, Polston EJ, Kloepper JW (2000) Plant growth-promoting rhizobacterial mediated protection in tomato against tomato mottle virus. Plant Dis 84:779–784
Nabity PD, Zavala JA, DeLucia EH (2008) Indirect suppression of photosynthesis on individual leaves by arthropod herbivory. Ann Bot 103:655–663
Ng JCK, Tian T, Falk BW (2004) Quantitative parameters determining whitefly (Bemisia tabaci) transmission of lettuce infectious yellows virus and an engineered defective RNA. J Gen Virol 86:2697–2707
Oliveira MRV, Henneberry TJ, Anderson P (2001) History, current status and collaborative research projects for Bemisia tabaci. Crop Prot 20:709–723
Park KS, Kloepper JW (2000) Activation of PR-1a promoter by rhizobacteria that induce systemic resistance in tobacco against Pseudomonas syringae pv. tabaci. Biol Control 18:2–9
Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF (2007) Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318:113–116
Pieterse CMJ, Dicke M (2007) Plant interactions with microbes and insects: From molecular mechanisms to ecology. Trends Plant Sci 12:564–569
Pieterse CMJ, Van Wees SCM, Hoffland E, Van Pelt JA, Van Loon LC (1996) Systemic resistance in Arabidopsis induced by biocontrol bacteria is independent of salicylic acid accumulation and pathogenesis-related gene expression. Plant Cell 8:1225–1237
Pieterse CMJ, Van Wees SCM, Van Pelt JA, Knoester M, Laan R, Gerrits H, Weisbeek PJ, Van Loon LC (1998) A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10:1571–1580
Pieterse CMJ, Van Wees SCM, Ton J, Van Pelt JA, Van Loon LC (2002) Signalling in rhizobacteria-induced systemic resistance in Arabidopsis thaliana. Plant Biol 4:535–544
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-PLUS. Springer, New York
Pontier D, Tronchet M, Rogowsky P, Lam E, Roby D (1998) Activation of hsr203, a plant gene expressed during incompatible plant-pathogen interactions, is correlated with programmed cell death. Mol Plant Microbe Interact 11:544–554
Raudenbush SW, Bryk AS (2002) Hierarchical linear models: applications and data analysis methods, 2nd edn. Sage, Thousand Oaks
Reymond P, Farmer EE (1998) Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol 1:404–411
Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, Fluhr R (2004) Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell 16:616–628
Sánchez-Hernández C, López MG, Délano-Frier JP (2006) Reduced levels of volatile emissions in jasmonate-deficient spr2 tomato mutants favour oviposition by insect herbivores. Plant Cell Environ 29:546–557
Sato M, Mitra RM, Coller J, Wang D, Spivey NW, Dewdney J, Denoux C, Glazebrook J, Katagiri F (2007) A high-performance, small-scale microarray for expression profiling of many samples in Arabidopsis-pathogen studies. Plant J 49:565–577
Schoch GA, Nikov GN, Alworth WL, Werck-Reichhart D (2002) Chemical inactivation of the cinnamate 4-hydroxylase allows for the accumulation of salicylic acid in elicited cells. Plant Physiol 130:1022–1031
Shi L, Jones WD, Jensen RV, Harris SC, Perkins RG, Goodsaid FM, Guo L, Croner LJ, Boysen C, Fang H, Qian F, Amur S, Bao W, Barbacioru CC, Bertholet V, Cao XM, Chu T-M, Collins PJ, Fan X-H, Frueh FW, Fuscoe JC, Guo X, Han J, Herman D, Hong H, Kawasaki ES, Li Q-Z, Luo Y, Ma Y, Mei N, Peterson RL, Puri RK, Shippy R, Su Z, Sun YA, Sun H, Thorn B, Turpaz Y, Wang C, Wang SJ, Warrington JA, Willey JC, Wu J, Xie Q, Zhang L, Zhang L, Zhong S, Wolfinger RD, Tong W (2008) The balance of reproducibility, sensitivity, and specificity of lists of differentially expressed genes in microarray studies. BMC Bioinformatics 9(Suppl 9):S10
Summers CG, Estrada D (1996) Chlorotic streak of bell pepper: a new toxicogenic disorder induced by feeding of the silverleaf whitefly, Bemisia argentifolii. Plant Dis 80:822
Teixeira J, Pereira S, Cánovas F, Salema R (2005) Glutamine synthetase of potato (Solanum tuberosum L. cv. Désirée) plants: cell- and organ-specific expression and differential developmental regulation reveal specific roles in nitrogen assimilation and mobilization. J Exp Bot 56:663–671
Thompson GA, Goggin FL (2006) Transcriptomics and functional genomics of plant defence induction by phloem-feeding insects. J Exp Bot 57:755–766
Thompson AR, Vierstra RD (2005) Autophagic recycling: lessons from yeast help define the process in plants. Curr Opin Plant Biol 8:165–173
Ton J, Van Pelt JA, Van Loon LC, Pieterse CMJ (2002) Differential effectiveness of salicylate-dependent and jasmonate/ethylene-dependent induced resistance in Arabidopsis. Mol Plant Microbe Interact 15:27–34
Tusher VG, Tibshirani R, Chu G (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98:5116–5121
van de Ven WTG, LeVesque CS, Perring TM, Walling LL (2000) Local and systemic changes in squash gene expression in response to silverleaf whitefly feeding. Plant Cell 12:1409–1423
Van der Ent S, Verhagen BWM, Van Doorn R, Bakker D, Verlaan MG, Pel MJC, Joosten RG, Proveniers MCG, Van Loon LC, Ton J, Pieterse CMJ (2008) MYB72 is required in early signaling steps of rhizobacteria-induced systemic resistance in Arabidopsis. Plant Physiol 146:1293–1304
Van Hulten M, Pelser M, van Loon LC, Pieterse CMJ, Ton J (2006) Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci USA 103:5602–5607
Van Loon LC, Bakker PAHM, Pieterse CMJ (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36:453–483
Van Oosten VR, Bodenhausen N, Reymond P, Van Pelt JA, Van Loon LC, Dicke M, Pieterse CMJ (2008) Differential effectiveness of microbially induced resistance against herbivorous insects in Arabidopsis. Mol Plant Microbe Interact 21:919–930
Van Peer R, Niemann GJ, Schippers B (1991) Induced resistance and phytoalexin accumulation in biological control of fusarium wilt of carnation by Pseudomonas sp. strain WCS417r. Phytopathology 81:728–733
Van Wees SCM, Pieterse CMJ, Trijssenaar A, Van’t Westende YAM, Hartog F, Van Loon LC (1997) Differential induction of systemic resistance in Arabidopsis by biocontrol bacteria. Mol Plant Microbe Interact 10:716–724
van Wees SCM, de Swart EAM, van Pelt JA, van Loon LC, Pieterse CMJ (2000) Enhancement of induced disease resistance by simultaneous activation of salicylate- and jasmonate-dependent defense pathways in Arabidopsis thaliana. Proc Natl Acad Sci USA 97:8711–8716
Verhagen BWM, Glazebrook J, Zhu T, Chang H-S, Van Loon LC, Pieterse CMJ (2004) The transcriptome of rhizobacteria-induced systemic resistance in Arabidopsis. Mol Plant Microbe Interact 17:895–908
Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19:195–216
Walling LL (2008) Avoiding effective defenses: strategies employed by phloem-feeding insects. Plant Physiol 146:859–866
Zarate SI, Kempema LA, Walling LL (2007) Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol 143:866–875
Zehnder G, Kloepper JW, Yao C, Wei G (1997a) Induction of systemic resistance in cucumber against cucumber beetles (Coleoptera: Chrysomelidae) by plant growth-promoting rhizobacteria. Biol Microb Control 90:391–396
Zehnder G, Kloepper JW, Tuzun S, Yao C, Wei G, Chambliss O, Shelby R (1997b) Insect feeding on cucumber mediated by rhizobacteria-induced plant resistance. Entomol Exp Appl 83:81–85
Zehnder GW, Yao C, Murphy JF, Sikora EJ, Kloepper JW (2000) Induction of resistance in tomato against cucumber mosaic cucumovirus by plant growth-promoting rhizobacteria. Biocontrol 45:127–137
Zehnder GW, Murphy JF, Sikora EJ, Kloepper JW (2001) Application of rhizobacteria for induced resistance. Eur J Plant Pathol 107:39–50
Zhang S, Reddy MS, Kloepper JW (2002a) Development of assays for assessing induced systemic resistance by plant growth-promoting rhizobacteria against blue mold of tobacco. Biol Control 23:79–86
Zhang S, Moyne AL, Reddy MS, Kloepper JW (2002b) The role of salicylic acid in induced systemic resistance elicited by plant growth-promoting rhizobacteria against blue mold of tobacco. Biol Control 25:288–296
Zhao Y, Thilmony R, Bender CL, Schaller A, He SY, Howe GA (2003) Virulence systems of Pseudomonas syringae pv. tomato promote bacterial speck disease in tomato by targeting the jasmonate signaling pathway. Plant J 36:485–499
Zhao H, Lu J, Lu S, Zhou Y, Wei J, Song Y, Wang T (2005) Isolation and functional characterization of a cinnamate 4-hydroxylase promoter from Populus tomentosa. Plant Sci 168:1157–1162
Zhu-Salzman K, Salzman RA, Ahn JE, Koiwa H (2004) Transcriptional regulation of sorghum defense determinants against a phloem-feeding aphid. Plant Physiol 134:420–431
Zhu-Salzman K, Bi J-L, Liu T-X (2005) Molecular strategies of plant defence and insect counter-defence. Insect Sci 12:3–15
Zonia LE, Stebbins NE, Polacco JC (1995) Essential role of urease in germination of nitrogen-limited Arabidopsis thaliana seeds. Plant Physiol 107:1097–1103
Acknowledgments
This work was supported by two postgraduate scholarships (165105, to MGEH and 182416, to JHVS) granted by The National Council for Science and Technology (CONACyT, México). We are grateful to Dr. Víctor Olalde Portugal for the provision of the BsDN PGPR.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Valenzuela-Soto, J.H., Estrada-Hernández, M.G., Ibarra-Laclette, E. et al. Inoculation of tomato plants (Solanum lycopersicum) with growth-promoting Bacillus subtilis retards whitefly Bemisia tabaci development. Planta 231, 397–410 (2010). https://doi.org/10.1007/s00425-009-1061-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-009-1061-9