Abstract
In cyanobacteria, photorespiratory 2-phosphoglycolate (2PG) metabolism is mediated by three different routes, including one route involving the glycine decarboxylase complex (Gcv). It has been suggested that, in addition to conversion of 2PG into non-toxic intermediates, this pathway is important for acclimation to high-light. The photoreduction of O2 (Mehler reaction), which is mediated by two flavoproteins Flv1 and Flv3 in cyanobacteria, dissipates excess reductants under high-light by the four electron-reduction of oxygen to water. Single and double mutants defective in these processes were constructed to investigate the relation between photorespiratory 2PG-metabolism and the photoreduction of O2 in the cyanobacterium Synechocystis sp. PCC 6803. The single mutants Δflv1, Δflv3, and ΔgcvT, as well as the double mutant Δflv1/ΔgcvT, were completely segregated but not the double mutant Δflv3/ΔgcvT, suggesting that the T-protein subunit of the Gcv (GcvT) and Flv3 proteins cooperate in an essential process. This assumption is supported by the following results: (1) The mutant Δflv3/ΔgcvT showed a considerable longer lag phase and sometimes bleached after shifts from slow (low light, air CO2) to rapid (standard light, 5% CO2) growing conditions. (2) Photoinhibition experiments indicated a decreased ability of the mutant Δflv3/ΔgcvT to cope with high-light. (3) Fluorescence measurements showed that the photosynthetic electron chain is reduced in this mutant. Our data suggest that the photorespiratory 2PG-metabolism and the photoreduction of O2, particularly that catalyzed by Flv3, cooperate during acclimation to high-light stress in cyanobacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria evolved about 3.5 billion years ago and were the first to perform oxygenic photosynthesis. These organisms are considered the ancestors of plant chloroplast (e.g., Deusch et al. 2008). The oxygen produced is, in fact, toxic for photosynthetic organisms, particularly under high-light conditions, because oxygen can serve as an acceptor of excess electrons generating reactive oxygen species (ROS). In addition to their general damaging effect, in photosynthetic organisms the reaction center protein D1 of photosystem II (PSII) is the preferential target of ROS. A higher rate of D1 protein destruction than repair at high-light leads to photoinhibition (Aro et al. 1993; Nishiyama et al. 2001; Takahashi et al. 2007). In addition, molecular oxygen competes with CO2 as a substrate for RubisCO and thereby lowers the carboxylation reaction and forms the toxic intermediate 2-phosphoglycolate (2PG), which inhibits Calvin–Benson cycle enzyme activities. 2PG is rapidly metabolized by the photorespiratory 2PG-metabolism. For scavenging of 2PG and other toxic compounds, it employs at least ten different enzymes in higher plants (Ogren 1984; Tolbert 1997; Bauwe and Kolukisaoglu 2003).
During their evolution, photosynthetic organisms adapted to the oxygen-containing environment and developed several strategies for acclimation to high-light. Over-reduction of the electron chain is initially avoided by the dissipation of excess absorbed light energy from the chlorophylls, mainly via carotenoids and other non-photochemical quenching (NPQ) mechanisms (Havaux et al. 2005; Kirilovsky 2007). In addition, a substantial part of electrons can be transferred from photosystem I (PSI) to molecular oxygen, which results in photoreduction of O2 via superoxide anion to H2O2 in plant chloroplasts, i.e., the Mehler reaction (Mehler 1951; Asada 1999). The produced ROS are quickly detoxified by the combined action of superoxide dismutase and peroxidases. Thereby, the photoreduction of O2 acts as electron sink under certain conditions, where up to 30% of the electrons from the light reactions can be directed to oxygen (Helman et al. 2005). Accordingly, it helps to prevent PSII from photodamage and is regarded as an important protection system in all photosynthetic organisms (Asada 1999; Badger et al. 2000; Helman et al. 2003).
Intriguingly, the photoreduction of O2 in cyanobacteria is quite different from that of plants. For the model cyanobacterium Synechocystis sp. PCC 6803 (hereafter Synechocystis), it was shown that O2 is reduced directly to water in one reaction mediated by A-type flavoproteins (Vicente et al. 2002; Helman et al. 2003). A-type flavoproteins, also referred to as flavodiiron proteins, are module proteins consisting of an N-terminal flavodoxin-like module (binding FMN) and a beta-lactamase module (harboring the non-heme diiron active site) as core modules (Wasserfallen et al. 1998; Frazão et al. 2000; Vicente et al. 2002). Cyanobacterial flavoproteins contain an additional C-terminal NAD(P)H:flavin oxidoreductase domain and are able to couple the NAD(P)H oxidation with the substrate reduction without an additional redox partner (reviewed in Vicente et al. 2008). The genome of Synechocystis encodes four putative A-type flavoproteins, but only two of them, Flv1 (Sll1521) and Flv3 (Sll0550), are apparently involved in light-dependent O2 reduction activity (Helman et al. 2003). Accordingly, the mutants Δflv1 and Δflv3 lack light-enhanced O2 consumption and hence the flavoproteins Flv1 and Flv3 are suggested to catalyze the cyanobacterial photoreduction of O2 (Helman et al. 2003). Recently, a role in the photoprotection of PSII has been shown for the two other Synechocystis flavoproteins, Flv2 and Flv4 (Zhang et al. 2009).
Photosynthetic CO2 assimilation represents the main acceptor for electrons from the photosynthetic water cleavage system. However, under CO2-limiting conditions the Calvin–Benson cycle activity is strongly reduced and oxygenase activity of RubisCO increases. The photorespiratory 2PG-metabolism recycles 75% of the organic carbon from 2PG and hence helps to avoid depletion of Calvin–Benson cycle intermediates (Osmond 1981; Wingler et al. 2000). Due to the operation of the efficient inorganic carbon concentrating mechanism (as reviewed in Kaplan and Reinhold 1999; Giordano et al. 2005; Badger et al. 2006), it was assumed that cyanobacteria do not possess a photorespiratory 2PG-metabolism (reviewed in Colman 1989). In contrast to this earlier view, we could recently show that an active photorespiratory 2PG-metabolism exists in Synechocystis, employing a plant-like 2PG-cycle, a bacterial-like glycerate pathway, and complete decarboxylation of glyoxylate via formate (Eisenhut et al. 2008). While defects in one or two of these metabolic branches only cause reduced growth under low-CO2 (0.035% CO2) conditions (Hagemann et al. 2005; Eisenhut et al. 2006), the complete loss of all three pathways in such Synechocystis mutants leads to a high-CO2-requiring-phenotype and highlights the essential function of photorespiratory 2PG-metabolism for cyanobacteria despite the carbon concentrating mechanism (Eisenhut et al. 2008).
For higher plants, it has been demonstrated that the photorespiratory 2PG-metabolism also plays a crucial role in high-light acclimation, since it helps to regenerate the acceptors, NADP+ and ADP, for ongoing reduction and energy storage, respectively, under excess light energy and/or lack of CO2 (Kozaki and Takeba 1996). Accordingly, mutants affected in the photorespiratory 2PG-metabolism showed depletion of Calvin–Benson cycle intermediates, which resulted in decreased consumption of ATP and NADPH (Wingler et al. 2000; Takahashi et al. 2007). While the cooperation of photorespiratory 2PG-metabolism and photoreduction of O2 in acclimation to high-light has been investigated in plants, the relation between these two oxygen-consuming mechanisms has not been investigated in cyanobacteria. In this work, we used Synechocystis mutants impaired in the photorespiratory 2PG-metabolism and in the photoreduction of O2, respectively, to address this question. Our results indicate that in cyanobacteria also these two pathways cooperate in the acclimation to high-light.
Materials and methods
Strains and culture conditions
The glucose-tolerant strain of Synechocystis sp. PCC 6803 was obtained from Prof. N. Murata (National Institute for Basic Biology, Okazaki, Japan) and served as the wild type (WT). Axenic cultures were grown on agar-solidified BG11 medium (Rippka et al. 1979) plates buffered with 20 mM TES–KOH to pH 8.0 at 30°C, under constant illumination (30 μmol photons m−2 s−1). Transformants were initially selected on media containing either 10 mg l−1 kanamycin, 4 mg l−1 spectinomycin or 5 mg l−1 chloramphenicol, but the segregation of clones and cultivation of mutants were performed either at 50 mg l−1 kanamycin, 20 mg l−1 spectinomycin, or 15 mg l−1 chloramphenicol. For the physiological characterization under standard conditions, axenic cultures (OD750 0.8–1.0, about 107 cells ml−1) were grown photoautotrophically in batch cultures (3 cm glass vessels with 5 mm glass tubes for aeration) at 29°C under continuous illumination at 165 μmol photons m−2 s−1 (warm light, Osram L58 W32/3, Munich, Germany) with bubbling of air enriched with CO2 (5% CO2 in air designated HC) in BG11 medium at pH 8.0. Pre-cultivation under slow growing conditions was performed in shaken Erlenmeyer flasks at low light of 50 μmol photons m−2 s−1 and at air level of CO2 (designated LC). For microarray analyses, cells were grown photoautotrophically in BG11 medium at pH 7.0 with bubbling of air enriched with CO2 (5% CO2 in air designated HC).
Growth was monitored by measurements of the OD at 750 nm. Photosynthetic pigment concentrations were measured and corrected as described by Huckauf et al. (2000). Absence of contamination by heterotrophic bacteria was checked by spreading 0.2 ml of culture on LB plates.
Generation of mutants
In order to generate mutations in the selected genes, interposon mutagenesis was applied by insertion of drug resistance cartridges against antibiotics into the coding sequences at selected restriction sites. The construction of the single mutants Δflv1 (sll1521::Cm), Δflv3 (sll0550::Sp) and ΔgcvT (sll0171::Km) was already described by Helman et al. (2003) and Hagemann et al. (2005), respectively. The double mutants Δflv1/ΔgcvT, Δflv3/ΔgcvT, and ΔgcvT/Δflv3 were raised using the original constructs for a second transformation of the single mutants Δflv1, Δflv3 or ΔgcvT. The genotype of the mutants was confirmed by PCR using total chromosomal DNA isolated from mutant clones and gene-specific primers (Table 1). Total DNA from Synechocystis strains was isolated according to Hagemann et al. (1997).
Conditions for photodamage
Strains were pre-cultivated for 2–5 days under standard conditions. For the photoinhibition experiments, concentrated cells at 10 μg Chl ml−1 were incubated for 30 min under high-light (1,400 μmol photons m−2 s−1) to induce photodamage. Subsequently, cells were transferred back to standard light (165 μmol photons m−2 s−1) and the repair was followed for 30 min. The high-light was provided by six standard fluorescence lamps (warm light, Osram L18 W32 and W76) and one strong light source (SOL 500/III Nr. 930044, Dr. Hoenle AG, Munich, Germany). For the photodamaging experiments, cells were incubated in smaller culture tubes (1.5 cm diameter with 5 mm glass tubes for aeration) at 29°C. In some experiments the de novo synthesis of proteins was blocked by 250 μg ml−1 lincomycin (Fluka, Sigma-Aldrich Chemie, Munich, Germany), which was added 1 min before the incubation under strong light. At defined time points, cells from 700 μl culture suspension were harvested by centrifugation (60 s at 2,000g at room temperature). The pellets were suspended in 350 μl BG11 to obtain 20 μg Chl ml−1 and used immediately for PAM measurements.
PAM measurements
Fluorescence measurements were performed with a modulated fluorometer (PAM-210, Walz, Effeltrich, Germany) using the saturation pulse method (Schreiber et al. 1995; Schreiber 1997). Strains were pre-cultivated for 2–5 days under standard conditions. For PAM measurements, cell suspensions were adjusted to 20 μg Chl ml−1 and incubated in the dark for a minimum of 30 min. Dark-adapted cells were illuminated with measuring red light (665 nm) at 0.2 μmol m−2 s−1 for 65 s (ML; dark) followed by red actinic light (650 nm) at 110 μmol m−2 s−1 for 150 s (AL; light) to measure F 0 (ML) and F s (AL), respectively (for nomenclature see van Kooten and Snel 1990). At defined time points, saturating pulses (3 μs, 3,500 μmol m−2 s−1) were applied to measure F m (ML) and Fm′ (AL) (Schreiber 1997). F 0 and F s values were used to calculate the increase of fluorescence (F s − F 0) from dark to light in WT and mutant cells.
In order to estimate the maximum PSII yield [F v/F m = (F m − F 0)/F m] after strong light the cells were adjusted to 20 μg Chl ml−1 in the dark (about 2 min) and subsequently illuminated for 70 s with measuring red light (665 nm) at 0.2 μmol m−2 s−1 to measure F 0. F m was monitored during repeated saturating pulses (3 μs, 3,500 μmol m−2 s−1) at intervals of 10 s. The average F m of five saturating pulses was used to calculate the maximal PSII yield (Schreiber 1997; Takahashi et al. 2007).
RNA-isolation and DNA-microarray analyses
Cells from 10 ml of culture were harvested by centrifugation at 2,860g for 5 min at 4°C and were immediately frozen at −80°C. Total RNA was extracted after pre-treatment with hot phenol and chloroform using the High-Pure RNA isolation kit (Roche Diagnostics, Mannheim, Germany). Direct cDNA labeling was done using the fluorescent dye either Cy3 or Cy5 (Amersham, GE Healthcare, Munich, Germany). Labeled cDNA was hybridized to 60-mer oligonucleotide DNA microarrays (Agilent, Amstelveen, The Netherlands) designed from the complete Synechocystis genome sequence. The whole procedure is described in detail by Eisenhut et al. (2007). Given values are the means and standard deviations of at least two independent experiments using RNA isolated from separate cultures. Inductions of 1.75-fold and repressions of 0.5-fold represented significant expression changes and were taken into consideration. The complete data set of microarray experiments is given as Supplementary Material.
Protein isolation and immuno-blotting
Cells from 50 ml of culture were harvested by centrifugation at 5,300g for 2 min at 4°C and were immediately frozen at −80°C. For protein isolation the pellets were re-suspended in 500 μl of 0.01 M HEPES buffer (pH 7.3) supplemented with 10 mM phenylmethylsulfonyl fluoride and sonicated (2 × 1 min, 35 W) under ice cooling. Total protein extracts (3 μg each) were separated in denaturating gels and used for immuno-blotting analyses (Eisenhut et al. 2007). A specific antibody was used against the orange carotenoid protein (OCP) from Synechocystis (dilution 1:1,250; received from Dr D. Kirilovsky, CNRS, France). Horseradish peroxidase (HRP) conjugated anti-rabbit IgG (Bio-Rad, Munich, Germany) was used as the secondary antibody.
Results
Generation and characterization of double mutants
In order to examine whether the photorespiratory 2PG-metabolism is linked to high-light acclimation in cyanobacteria like in plants, we generated Synechocystis double mutants with an impaired photoreduction of O2 as well as an impaired photorespiratory 2PG-metabolism. For this purpose, the mutants Δflv1 and Δflv3 (Helman et al. 2003) defective in the photoreduction of O2 were transformed with a DNA construct bearing an inactivated gcvT gene encoding the T-protein subunit of the glycine decarboxylase complex (Gcv, Hagemann et al. 2005). The T-protein subunit produces NH4 + and methylene-tetrahydrofolate from the aminomethyl-group of glycine bound to the H-protein subunit of Gcv (Bauwe and Kolukisaoglu 2003). Putative Δflv1/ΔgcvT and Δflv3/ΔgcvT double mutants were selected as chloramphenicol/kanamycin- and spectinomycin/kanamycin-resistant clones, respectively. Characterization of their genotypes by PCR analyses showed complete segregation of the three single mutants and of the double mutant Δflv1/ΔgcvT. All the WT copies of these genes were inactivated by the relevant cartridges leading to larger PCR fragments (Fig. 1). In contrast, the double mutant Δflv3/ΔgcvT still retained WT copies of gcvT that were amplified in addition to the mutated gene fragment. Incomplete segregation of the double mutant Δflv3/ΔgcvT was detected in several independently obtained clones, even after many generations of growth under selective conditions. This unexpected behavior was also observed when we used the single mutant ΔgcvT as the parental strain and transformed it with an inactivated flv3 gene; in this case the cells maintained WT copies of flv3 (data not shown).
Genotypic characterization of the Synechocystis single mutants Δflv1 and Δflv3 defective in the photoreduction of O2, ΔgcvT blocked in the photorespiratory 2PG-metabolism, as well as double mutants Δflv1/ΔgcvT and Δflv3/ΔgcvT defective in both processes by PCR. For the PCR reactions total DNA of the mentioned strains (upper line) and the gene-specific primers (lower line) were used as given in Table 1. (Abbreviations and expected fragment sizes: M, length marker λ-DNA EcoRI/HindIII; WT: 1.6 kb for flv1, 0.9 kb for flv3 and 1.9 kb for gcvT. The sizes of the mutated genes after insertions of drug resistance cartridges are: 2.0 kb for flv1::Cm, 2.6 kb for flv3::Sp and 2.2 kb for gcvT::Km)
The fact that it was not possible to combine the two mutations in gcvT and flv3 in one cell provided the first indication of a functional relationship between photorespiratory 2PG-metabolism and the photoreduction of O2 in a cyanobacterium. This result was unexpected since in previous studies the single mutant ΔgcvT showed only a little reduction of growth under low-CO2 conditions (Hagemann et al. 2005; Eisenhut et al. 2006) and the single mutant Δflv3 behaved also similar to WT cells despite the defect in the photoreduction of O2 (Helman et al. 2003). Moreover, these results indicated that the Flv3 protein seems to be more important than the Flv1 protein, at least when combined with a mutation in glycine decarboxylase. This view is also supported by expression analyses, in which only flv3, but not flv1, was found to be strongly up-regulated after transfer of the cells from a high to a low level of CO2 (Wang et al. 2004; Eisenhut et al. 2007).
Microarray analyses
We performed these analyses in order to characterize the effect of a mutated flv3 gene on global gene expression in Synechocystis. Similar experiments were performed previously with the single mutant ΔgcvT (Eisenhut et al. 2007). The complete data set of microarray experiments is given as supplementary material. Despite the observed changes in its phenotype (see below), only a few genes showed significant expression changes in mutant Δflv3 as compared to WT. In general, the up-regulated genes encoded for three groups of proteins (Table 2). The first group comprises proteins known to respond to various stress treatments and can perhaps be regarded as general stress responsive (e.g., Sll1862-1863, HtrA, HspA, SigH, RepA) (Los et al. 2008). The second group is involved in the homeostasis of metals such as iron, zinc, and cobalt (ZiaA, CoaT, SufBCS, FutC). The third group of proteins might be necessary to cope with the alterations in electron flow such as NdhD2, OCP, and the thioredoxin-like AhpC (Ohkawa et al. 2000; Kobayashi et al. 2004; Wilson et al. 2006). It is noteworthy that none of the genes encoding proteins involved in 2PG-metabolism (e.g., gcvT) or in the acclimation to low-CO2 displayed significant changes in its expression level in the mutant Δflv3, as compared to WT, under standard conditions. The expression of the second flavoprotein flv1 involved in the photoreduction of O2 was also not affected in the mutant Δflv3.
Among the down-regulated genes (Table 3) we found genes encoding for subunits of the phycobilisome (cpcA, B, C1, C2, D; apcA, B), which is in accordance with the up-regulation of nblA1 gene for the protein responsible for their degradation (Baier et al. 2001). Moreover, many genes for subunits of PSI (psaA, B, C, D, E, J, L) decreased about twofold in mutant Δflv3 as compared to WT (Table 3), while no or only slight changes were observed in the abundance of transcripts for PSII subunits. In addition, many genes for proteins of unknown function were down-regulated in mutant Δflv3.
Effects of light intensity and CO2 level on growth
While characterizing changes in the phenotypes of the single and double mutants we observed that particularly the mutants affected in flv3 were extremely sensitive when transferred from slow (50 μmol photons m−2 s−1, 0.035% CO2, routinely used for pre-cultivation) to rapid growth conditions (165 μmol photons m−2 s−1, 5% CO2). In order to investigate this behavior in more detail, cells were transferred from the pre-culture into the CO2-gassed culture system under defined conditions. The ability of the various strains to acclimate to the new environment was strongly affected by the initial cell density (equivalent to different actual light intensities for the cell suspension). When applying standard growth conditions, i.e., inoculum densities of OD750 = 0.8–1.0, both the WT and mutants were able to acclimate to the new conditions. In contrast, when the initial cell density was reduced (OD750 = 0.2), mutants ΔgcvT, Δflv3, and Δflv3/ΔgcvT showed a significantly longer lag phase as compared to WT, appeared yellowish and sometimes even bleached after 46 h of cultivation (Fig. 2a, b). Under these conditions, mutants Δflv1 and even Δflv1/ΔgcvT behaved similarly to WT.
Acclimation of cells of the Synechocystis WT and defined mutants to a shift from slow to rapid growth conditions. a The representative growth curves are shown (increase in OD at 750 nm) of cells observed after the transfer of cells from shaken Erlenmeyer cultures at 50 μmol photons m−2 s−1 and air level of CO2 into the standard cultivation system with 165 μmol photons m−2 s−1 and high CO2 (5% CO2). All strains were inoculated with a relatively low OD750 of 0.2. Each point represents the average from at least three independent experiments with standard deviations. b Optical appearance of the cultures after incubation of the cells under the new growth conditions for 46 h. Please note that the pictures were taken from one typical experiment. The average pigment changes are displayed in Table 4
Furthermore, the differences observed under transient situations were also apparent under steady state conditions. Growth rates of the single mutants ΔgcvT and Δflv3 and particularly of the double mutant Δflv3/ΔgcvT were reduced under high- or low-CO2 level as compared to the WT and the single mutant Δflv1 (Table 4). The strains exhibiting reduced growth appeared yellowish due to a reduction in the Chl content and an increased carotenoid level (Table 4). The latter findings are in agreement with our microarray data, where genes for PSI binding the majority of Chl were strongly reduced and the ocp gene was up-regulated (Tables 2, 3).
Characterization of Chl fluorescence parameters
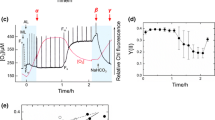
Results presented in Fig. 2 suggested higher sensitivity to high-light conditions in mutants bearing a combined defect in the photorespiratory 2PG-metabolism and the photoreduction of O2, which could be linked to the activity of the PSII. Fluorescence parameters are often used to assess PSII activity and photoinhibition in plants and cyanobacteria (e.g., Krause and Weis 1991; Campbell et al. 1998). We applied a defined PAM measuring protocol which consisted of three illumination stages and several flashes with saturating light to obtain the fluorescence parameters (Fig. 3; for nomenclature see van Kooten and Snel 1990; Campbell et al. 1998).
Changes in fluorescence levels induced by different light levels measured in a PAM fluorometer using the saturation pulse method. Dark-adapted cell suspensions of wild type (a) and double mutant Δflv3/ΔgcvT (b) at the same Chl contents (20 μg Chl ml−1) were illuminated with ML (dark) for 65 s followed by AL (light) for 150 s to measure the minimal fluorescence F 0 (ML) and the steady state fluorescence F s (AL). To estimate the maximal fluorescence in the dark (F m) and actinic light (F m′), saturating pulses of 3 μs were applied. The minimal fluorescence F 0 was calculated from values obtained after 10–12 s (before the first saturating pulse), while the steady state fluorescence F s was calculated from values around 210–212 s (after the last saturating pulse during actinic light). F m values are the average from all maximal fluorescence values measured after saturating light pulses in the presence of measuring light. c The increase in fluorescence from F 0 to F s is shown. Each column and bar represents the average of five independent experiments. Statistically significant differences in the fluorescence increase compared to WT (asterisk) and corresponding single-mutant (double asterisk) cells
As observed previously (Helman et al. 2003), the maximal PSII yield (F v/F m) did not differ significantly between WT and mutants Δflv1, Δflv3, and ΔgcvT (Table 4). However, comparison of the rise in fluorescence from F 0 (ML) to F s (AL) revealed interesting differences (Fig. 3c). The single mutant ΔgcvT showed no alterations in F s as compared with the WT. In contrast, a considerably elevated F s (65 to 72% as compared to WT) was observed in the single mutants Δflv1 and Δflv3 (consistent with the results from Helman et al. 2003) and even more so in the double mutants Δflv1/ΔgcvT and Δflv3/ΔgcvT (115–160%) indicating a lower ability to oxidize the reduced PQ pool. The further increase of F s in the combined mutants defective in photorespiratory 2PG-metabolism and in photoreduction of O2 could indicate that both mechanisms operate as effective electron acceptors for the linear electron transport chain (Fig. 3c) in agreement with results obtained with higher plants (Kozaki and Takeba 1996; Takahashi et al. 2007).
Recently, the water-soluble OCP was implicated in quenching of phycobilisome excitation in high-light-treated cyanobacteria (Wilson et al. 2006). Our microarray analyses suggested a rise in the transcript abundance of the respective gene (Table 2). Using immuno-blot analyses, we investigated whether this effect is translated to the protein level. Corresponding to the transcriptional data, an increase in the quantity of OCP was detectable in all single and double mutants with interrupted flv3, whereas the mutant ΔgcvT exhibited a lower amount of OCP (Fig. 4) corresponding to the reduced ocp mRNA level (Eisenhut et al. 2007). We also examined the amount of the D1 and RubisCO proteins but did not observe significant alterations in their levels under the standard growth conditions (data not shown).
Immuno-blotting analyses with protein extracts from cells of the WT and mutants ΔgcvT, Δflv3 and Δflv3/ΔgcvT of Synechocystis, respectively, to determine the amount of the orange carotenoid protein OCP. The cells were cultivated under standard growth conditions. Three micrograms of total soluble protein was applied per lane on a SDS-PAGE gel. OCP was detected by a specific antibody via chemiluminescence
Recovery of maximal PSII yield after high-light treatment
In order to investigate the response of the various strains to excess light and recovery, we analyzed the level of maximal PSII yield F v/F m in WT and mutants (Fig. 5a). During exposure to high-light of 1,400 μmol photons m−2 s−1 for 30 min the maximal photochemical efficiency decreased to 25–35% (without lincomycin) and 5–15% (with lincomycin), respectively, in all strains used here as compared with the control, i.e., dark-incubated cells (Table 4). The rate of decline in maximal PSII yield reflects the balance between damage to the photochemical machinery and its repair. In order to eliminate repair processes we used lincomycin, which inhibits protein synthesis-dependent recovery. As expected, the rate of decline in the photochemical activity was faster in the presence of lincomycin but did not differ between the various strains used (Fig. 5b). We conclude that the drainage of electrons by the photoreduction of O2 and photorespiratory 2PG-metabolism (inhibited in the Δflv and ΔgcvT mutants, respectively) did not alter the damage caused by excess light. In contrast, while the WT and mutant ΔgcvT regained about 80% of the initial activity after 30 min recovery at standard light intensity, mutants impaired in the photoreduction of O2 recovered significantly slower (Fig. 5a). Here, too, the mutation in flv3 led to stronger effects than in flv1. In addition, the double mutant Δflv3/ΔgcvT could only reach 48% of the photochemical activity in the control cells within that time (Fig. 5a).
Photoinhibition experiments with cells of the Synechocystis WT and mutants ΔgcvT, Δflv3, Δflv1, ΔgcvT/Δflv1 and Δflv3/ΔgcvT. Maximal PSII yield expressed as the percentage of F v/F m [(F m − F 0)/F m] of dark controls was measured using cells exposed to high-light (1,400 μmol photons m−2 s−1) for 30 min and then incubated 30 min under standard light (165 μmol photons m−2 s−1) to allow the recovery of PSII a without and b with lincomycin treatment (250 μg ml−1), respectively. The average F m of five repeated saturating pulses (660 nm, 3 μs, 3,500 μmol m−2 s−1) in intervals of 10 s was used to calculate F v. Each point and bar represents the average from four independent cultivation experiments. The maximal PSII yields (F V/F m) at time point 0 were: WT—0.492 ± 0.039, ΔgcvT—0.548 ± 0.018, Δflv1—0.497 ± 0.035, Δflv3—0.562 ± 0.028, Δflv1/ΔgcvT—0.504 ± 0.044, Δflv3/ΔgcvT—0.547 ± 0.033
Discussion
We examined a possible cooperation between the photoreduction of O2 and the photorespiratory 2PG-metabolism in the acclimation of Synechocystis to high-light. Despite the fact that inactivation of both flv1 and flv3 resulted in complete arrest of the light-dependent O2 reduction, Flv3 is more important for light acclimation than Flv1. This is indicated by the severe inhibition of Δflv3 growth, but not Δflv1, after transfer to excess light. Our conclusion is also supported by the differential expression of flv1 and flv3: transcription of flv3 but not of flv1 increased under high-light and low-CO2 conditions (Hihara et al. 2001; Wang et al. 2004; Eisenhut et al. 2007).
Due to the clear inability to photoreduce O2 in both Δflv1 and Δflv3 mutants, it was proposed that the photoreduction of O2 is catalyzed by an Flv1–Flv3-heterodimer in vivo (Helman et al. 2003). The observed clear change in the phenotype of mutant Δflv3, in contrast to the missing effects of the mutation in flv1 (Fig. 2) raises the possibility that under these conditions, and in particular increased light stress, a homodimer of Flv3 could function in mutant Δflv1 as is the case with isolated Flv3, which exhibits NADPH-dependent O2 reduction in vitro (Vicente et al. 2002; Helman et al. 2003).
Our microarray analyses revealed that inactivation of flv3 resulted in an increased effect of excess light on the expression of genes known to be affected by such conditions in the WT (Hihara et al. 2001). The stressed status of mutant Δflv3 is indicated by the elevated expression of several stress proteins such as ndhD2 and ocp, which are normally induced by different environmental stresses (Los et al. 2008) and by the changes in expression of various genes encoding proteins involved in the metal homeostasis (Singh et al. 2003). On the other hand, expression of genes for phycobilisome and PSI subunits was considerably depressed in Δflv3 as compared with the WT (Tables 2, 3). Hihara et al. (2001) proposed that down-regulation of phycobilisome genes is likely to reduce the effective light-harvesting cross-section and, thus, helps minimizing the damage to PSII. Taken together, the transcript abundance data suggest that the Δflv3 mutant experiences a more severe stress at standard growth conditions than the WT.
The loss of the protein Flv3, which is responsible for the major activity in photoreduction of O2, caused a high-light-phenotype. This is not only illustrated by the transcriptional changes but also demonstrated by the physiological characteristics, particularly under transient conditions. Earlier studies (Helman et al. 2003) did not reveal a significant effect of the inactivation of the flv genes on the steady state growth parameters. In contrast, we found changes in the light acclimation of the Δflv3 mutant, but not in Δflv1, particularly following transitions in the growth conditions or initiation of growth at a low level of inoculum size. The Δflv3 mutant showed impaired ability to cope with these conditions (Fig. 2).
An important outcome from this study is that the various mechanisms that help dissipate excess light energy, such as CO2 cycling (Tchernov et al. 2003) or NPQ (Campbell et al. 1998; Wilson et al. 2006) or the action of Flv2 and Flv4 (Zhang et al. 2009) could not compensate for the loss of the photoreduction of O2 (Fig. 2). It was, therefore, surprising that the rate of decline in maximal PSII yield following exposure to excess light, often ascribed to PSII activity, was not affected by the mutations introduced here, including Δflv3, in either the absence or presence of a protein synthesis inhibitor (Fig. 5). This is in agreement with the results of Helman et al. (2003) who also reported that, despite the very large flux of electrons via the photoreduction of O2, flv3 inactivation hardly affected the extent of photoinhibition (assessed by the decline in F v). Naturally, in the absence of a protein synthesis inhibitor the decline in photosynthetic ability with time (Fig. 5) mirrors the balance between the damage to PSII and its repair. This is also reflected in the difference in the slopes obtained in the absence or presence of lincomycin (Fig. 5a, b, respectively).
In a recent study, Takahashi et al. (2007) suggested that photorespiratory 2PG-metabolism is involved in the repair of photoinhibitory damage in Arabidopsis. The fact that the rate of repair in a single ΔgcvT mutant was identical to that of the WT (Fig. 5a) did not lend support to this possibility in Synechocystis. On the other hand, the rate of repair was significantly reduced in mutants Δflv3 and Δflv3/ΔgcvT, suggesting that removal of electrons from the linear photosynthetic electron chain by the photoreduction of O2 may be involved in the repair.
Unexpectedly, the most severe phenotype in all the aspects examined here was observed in the double mutant Δflv3/ΔgcvT, despite the fact that it was not completely segregated and that a single mutation in gcvT did not produce a clear phenotype (Figs. 2, 5), since other routes for the photorespiratory 2PG-metabolism are intact (Eisenhut et al. 2006, 2008). Obviously, the plant-like route for 2PG-metabolism involving the Gcv is of highest importance for 2PG-metabolism and here for the dissipation of excess reductants, since the Gcv step releases CO2 and NH3 from glycine, which are re-assimilated using high amounts of NADPH2 and ATP. Phenotypic differences in non-segregated Synechocystis mutants have already been observed (e.g., Wang et al. 2002; Gutekunst et al. 2005; Oliveira and Lindblad 2008), suggesting that the reduced gene dosage certainly results in a lower content of the corresponding protein. Furthermore, growth inhibition was observed when the cells were transferred from a low-light intensity and air level of CO2 to standard-light intensity and 5% CO2. The elevated CO2 level would be expected to inhibit the oxygenase activity of RubisCO, but we previously found glycolate accumulation under such conditions in Synechocystis (Eisenhut et al. 2006, 2008). Nevertheless, the fact that mutant Δflv3/ΔgcvT exhibits the most severe phenotype supports the notion that the two processes, photoreduction of O2 and photorespiratory 2PG-metabolism, seem to cooperate in the dissipation of excess reducing equivalents and in the prevention of a low redox poise possibly by a mechanism with mutual functional replacement in the cells.
Abbreviations
- 2PG:
-
2-Phosphoglycolate
- AL:
-
Actinic light
- Car:
-
Carotenoids
- Chl:
-
Chlorophyll a
- F 0 :
-
Minimal fluorescence of dark-adapted cells
- Flv:
-
Flavoprotein
- F m/F m′:
-
Maximal fluorescence
- F s :
-
Fluorescence of actinic light adapted cells
- F v :
-
Variable fluorescence
- F v/F m :
-
Maximal PSII yield
- Gcv:
-
Glycine decarboxylase complex
- GcvT:
-
T-protein subunit of the glycine decarboxylase complex
- ML:
-
Measuring light
- NPQ:
-
Non-photochemical quenching
- OCP:
-
Orange carotenoid protein
- OD:
-
Optical density
- PC:
-
Phycocyanin
- PSI:
-
Photosystem I
- PSII:
-
Photosystem II
- ROS:
-
Reactive oxygen species
- RubisCO:
-
Ribulose-1,5-bisphosphate carboxylase/oxygenase
- SD:
-
Standard deviation
- WT:
-
Wild type
References
Aro EM, Virgin I, Andersson B (1993) Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta 1143:113–134
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Badger MR, von Caemmerer S, Ruuska S, Nakano H (2000) Electron flow in higher plants and algae: rates and control of direct photoreduction (Mehler reaction) and RubisCO oxygenase. Philos Trans R Soc Lond B Biol Sci 355:1433–1446
Badger MR, Price GD, Long BM, Woodger FJ (2006) The environmental plasticity and ecological genomics of the cyanobacterial CO2 concentrating mechanism. J Exp Bot 57:249–265
Baier K, Nicklisch S, Grundner C, Reinecke J, Lockau W (2001) Expression of two nblA-homologous genes is required for phycobilisome degradation in nitrogen-starved Synechocystis sp. PCC6803. FEMS Microbiol Lett 195:35–39
Bauwe H, Kolukisaoglu U (2003) Genetic manipulation of glycine decarboxylation. J Exp Bot 54:1523–1535
Campbell D, Hurry V, Clarke AK, Gustafsson P, Öquist G (1998) Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol Mol Biol Rev 62:667–683
Colman B (1989) Photosynthetic carbon assimilation and the suppression of photorespiration in the cyanobacteria. Aquat Bot 34:211–231
Deusch O, Landan G, Roettger M, Gruenheit N, Kowallik KV, Allen JF, Martin W, Dagan T (2008) Genes of cyanobacterial origin in plant nuclear genomes point to a heterocyst-forming plastid ancestor. Mol Biol Evol 25:748–761
Eisenhut M, Kahlon S, Hasse D, Ewald R, Lieman-Hurwitz J, Ogawa T, Ruth W, Bauwe H, Kaplan A, Hagemann M (2006) The plant-like C2 glycolate cycle and the bacterial-like glycerate pathway cooperate in phosphoglycolate metabolism in cyanobacteria. Plant Physiol 142:333–342
Eisenhut M, von Wobeser EA, Jonas L, Schubert H, Ibelings BW, Bauwe H, Matthijs HCP, Hagemann M (2007) Long-term response towards inorganic carbon limitation in wild type and glycolate turnover mutants of the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Physiol 144:1946–1959
Eisenhut M, Ruth W, Haimovich M, Bauwe H, Kaplan A, Hagemann M (2008) The photorespiratory glycolate metabolism is essential for cyanobacteria and might have been conveyed endosymbiontically to plants. Proc Natl Acad Sci USA 105:17199–17204
Frazão C, Silva G, Gomes CM, Matias P, Coelha R, Sieker L, Macedo S, Liu MY, Oliviera S, Teixeira M, Xavier AV, Rodrigues-Pousada C, Carrondo MA, Le Gall J (2000) Structure of a dioxygen reduction enzyme from Desulfovibrio gigas. Nat Struct Biol 7:1041–1045
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56:99–131
Gutekunst K, Phunpruch S, Schwarz C, Schuchardt S, Schulz-Friedrich R, Appel J (2005) LexA regulates the bidirectional hydrogenase in the cyanobacterium Synechocystis sp. PCC 6803 as a transcription activator. Mol Microbiol 58:810–823
Hagemann M, Schoor A, Jeanjean R, Zuther E, Joset F (1997) The stpA gene form Synechocystis sp. strain PCC 6803 encodes the glucosylglycerol-phosphate phosphatase involved in cyanobacterial osmotic response to salt shock. J Bacteriol 179:1727–1733
Hagemann M, Vinnemeier J, Oberpichler I, Boldt R, Bauwe H (2005) The glycine decarboxylase complex is not essential for the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Biol 7:15–22
Havaux M, Guedeney G, Hagemann M, Yeremenko N, Matthijs H, Jeanjean R (2005) The chlorophyll-binding protein IsiA is inducible by high light and protects the cyanobacterium Synechocystis PCC 6803 from photooxidative stress. FEBS Lett 579:2289–2293
Helman Y, Tchernov D, Reinhold L, Shibata M, Ogawa T, Schwarz R, Ohad I, Kaplan A (2003) Genes encoding A-type flavoproteins are essential for photoreduction of O2 in cyanobacteria. Curr Biol 13:230–235
Helman Y, Barkan E, Eisenstadt D, Luz B, Kaplan A (2005) Fractionation of the three stable oxygen isotopes by oxygen producing and consuming reactions in photosynthetic organisms. Plant Physiol 138:2292–2298
Hihara Y, Kamei A, Kanehisa M, Kaplan A, Ikeuchi M (2001) DNA microarray analysis of cyanobacterial gene expression during acclimation to high light. Plant Cell 13:793–806
Huckauf J, Nomura C, Forchhammer K, Hagemann M (2000) Stress responses of Synechocystis sp. strain PCC 6803 mutants impaired in genes encoding putative alternative sigma factors. Microbiology 146:2877–2889
Kaplan A, Reinhold L (1999) CO2 Concentrating mechanisms in photosynthetic microorganisms. Annu Rev Plant Physiol Plant Mol Biol 50:539–570
Kirilovsky D (2007) Photoprotection in cyanobacteria: the orange carotenoid protein (OCP)-related non-photochemical-quenching mechanism. Photosynth Res 93:7–16
Kobayashi M, Ishizuka T, Katayama M, Kanehisa M, Bhattacharyya-Pakrasi M, Pakrasi HB, Ikeuchi M (2004) Response to oxidative stress involves a novel peroxiredoxin gene in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol 45:290–299
Kozaki A, Takeba G (1996) Photorespiration protects C3 plants from photooxidation. Nature 384:557–560
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis—the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Los DA, Suzuki I, Zinchenko VV, Murata N (2008) Stress responses in Synechocystis: regulated genes and regulatory systems. In: Herrero A, Flores E (eds) The cyanobacteria: molecular biology, genomics and evolution. Caister Academic Press, Norfolk, pp 117–157
Mehler AH (1951) Studies on reactions of illuminated chloroplasts. I. Mechanisms of the reduction of oxygen and other Hill reagents. Arch Biochem Biophys 33:65–77
Nishiyama Y, Yamamoto H, Allakhverdiev SI, Inaba M, Yokota A, Murata N (2001) Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J 20:5587–5594
Ogren WL (1984) Photorespiration—pathways, regulation, and modification. Annu Rev Plant Physiol 35:415–442
Ohkawa H, Pakrasi HB, Ogawa T (2000) Two types of functionally distinct NAD(P)H dehydrogenases in Synechocystis sp. strain PCC6803. J Biol Chem 275:31630–31634
Oliveira P, Lindblad P (2008) An AbrB-like protein regulates the expression of the bidirectional hydrogenase in Synechocystis sp. strain PCC 6803. J Bacteriol 190:1011–1019
Osmond CB (1981) Photorespiration and photoinhibition: some implications for the energetics of photosynthesis. Biochim Biophys Acta 639:77–98
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Schreiber U (1997) Chlorophyllfluoreszenz und photosynthetische Energieumwandlung: Einfache einführende Experimente mit dem TEACHING-PAM Chlorophyll-Fluorometer, 1. Ausgabe Jan 1997, Heinz Walz GmbH, Effeltrich
Schreiber U, Endo T, Mi H, Asada K (1995) Quenching analysis of chlorophyll fluorescence by the saturation pulse method: particular aspects relating to the study of eukaryotic algae and cyanobacteria. Plant Cell Physiol 36:873–882
Singh AK, McIntyre LM, Sherman LA (2003) Microarray analysis of the genome-wide response to iron deficiency and iron reconstitution in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol 132:1825–1839
Takahashi S, Bauwe H, Badger M (2007) Impairment of the photorespiratory pathway accelerates photoinhibition of photosystem II by suppression of repair but not acceleration of damage processes in Arabidopsis. Plant Physiol 144:487–494
Tchernov D, Silverman J, Luz B, Kaplan A (2003) Massive light-dependent cycling of inorganic carbon between photosynthetic microorganisms and their surroundings. Photosynth Res 77:95–103
Tolbert NE (1997) The C-2 oxidative photosynthetic carbon cycle. Annu Rev Plant Physiol Plant Mol Biol 48:1–25
van Kooten O, Snel JFH (1990) The use of chlorophyll nomenclature in plant stress physiology. Photosynth Res 25:147–150
Vicente JB, Gomes CM, Wasserfallen A, Teixeira M (2002) Module fusion in an A-type flavoprotein from the cyanobacterium Synechocystis condenses a multiple-component pathway in a single polypeptide chain. Biochem Biophys Res Commun 294:82–87
Vicente JB, Justino MC, Gonçalves VL, Saraiva LM, Teixeira M (2008) Biochemical, spectroscopic, and thermodynamic properties of flavodiiron proteins. Methods Enzymol 437:21–45
Wang HL, Postier BL, Burnap RL (2002) Polymerase chain reaction-based mutageneses identify key transporters belonging to multigene families involved in Na+ and pH homeostasis of Synechocystis sp. PCC 6803. Mol Microbiol 44:1493–1506
Wang HL, Postier BL, Burnap RL (2004) Alterations in global patterns of gene expression in Synechocystis sp. PCC 6803 in response to inorganic carbon limitation and the inactivation of ndhR, a LysR family regulator. J Biol Chem 279:5739–5751
Wasserfallen A, Ragettli S, Jounneau Y, Leisinger T (1998) A family of flavoproteins in the domains Archaea and Bacteria. Eur J Biochem 254:325–332
Wilson A, Ajlani G, Verbavatz JM, Vass I, Kerfeld CA, Kirilovsky D (2006) A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria. Plant Cell 18:992–1007
Wingler A, Lea PJ, Quick WP, Leegood RC (2000) Photorespiration: metabolic pathways and their role in stress protection. Philos Trans R Soc Lond B Biol Sci 355:1517–1529
Zhang P, Allahverdiyeva Y, Eisenhut M, Aro EM (2009) Flavodiiron proteins in oxygenic photosynthetic organisms: photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803. PLoS One 4:e5331
Acknowledgments
The gift of the OCP antibody by Dr D. Kirilovsky (CNRS, Paris, France) is greatly acknowledged. The critical discussion of PAM data with Prof. H. Schubert (University Rostock) is highly appreciated. Many thanks to Dr Martijs Jonker (Microarray Department, University of Amsterdam, The Netherlands) for the help during DNA-microarray data evaluation. The technical assistance of Klaudia Michl is acknowledged. The work was supported by a grant from the DFG (Deutsche Forschungsgemeinschaft).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Hackenberg, C., Engelhardt, A., Matthijs, H.C.P. et al. Photorespiratory 2-phosphoglycolate metabolism and photoreduction of O2 cooperate in high-light acclimation of Synechocystis sp. strain PCC 6803. Planta 230, 625–637 (2009). https://doi.org/10.1007/s00425-009-0972-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-009-0972-9