Abstract
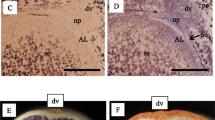
The nucellar projection (NP)/endosperm transfer cell (ETC) complex represents the link between maternal and filial seed tissues in barley and mediates nutrient transfer into the endosperm. Cells of NP function as metabolic interface to precondition amino acid supply of the endosperm. The organ displays a top-down gradient of differentiation, with mitotically active, differentiating/elongating as well as disintegrating cells, characterized by proteolysis and nitrogen remobilization. To understand metabolism, interconversion and transfer of amino acids at the maternal–filial boundary, we applied a combined transcriptome and metabolite approach based on laser-assisted microdissection. Results suggest that amino acid degradation observed in NP largely occurs within mitochondria, consistent with their role in controlling amino acid homeostasis and metabolism. Differentially expressed genes and free amino acid levels associated with glutamate and glutamine metabolism indicate concerted action of glutamine dehydrogenase, glutamine synthetase and alanine:glyoxylate aminotransferase 2 within a hypothetical cycle for glutamine and alanine degradation and re-synthesis of the preferred transport form glutamine. Stimulation of gene expression involved in methionine metabolism in NP suggests a pathway of regulated synthesis of S-methylmethionine and a possible mechanism for the transfer of reduced sulphur from maternal tissues into the endosperm. Thus, the established micromethods revealed strategies in NP of young barley grains for mobilization and metabolism of transient N and S reserves and transfer into the endosperm.




Similar content being viewed by others
Abbreviations
- Ala:
-
Alanine
- Arg:
-
Arginine
- Asp:
-
Aspartate
- Cys:
-
Cysteine
- GABA:
-
Gamma amino butyric acid
- Gln:
-
Glutamine
- Glu:
-
Glutamate
- Gly:
-
Glycine
- His:
-
Histidine
- Leu:
-
Leucine
- Lys:
-
Lysine
- Met:
-
Methionine
- Pro:
-
Proline
- SAM:
-
S-Adenosylmethionine
- Ser:
-
Serine
- Trp:
-
Tryptophan
- Val:
-
Valine
- amol:
-
Attomol (10−18 mol)
- DAF:
-
Days after flowering
- ETC:
-
Endosperm transfer cells
- LMPC:
-
Laser microdissection coupled to laser pressure catapulting
- NP:
-
Nucellar projection
- UPLC:
-
Ultra performance liquid chromatography
References
Angeles G, Berrio-Sierra J, Joseleau JP, Lorimier P, Lefebvre A, Ruel K (2006) Preparative laser capture microdissection and single-pot cell wall material preparation: a novel method for tissue-specific analysis. Planta 224:228–232
Balestrini R, Bonfante P (2008) Laser microdissection (LM): application to plant material. Plant Biosyst 142:331–336
Bernard SM, Møller AL, Dionisio G, Kichey T, Jahn TP, Dubois F, Baudo M, Lopes MS, Tercé-Laforgue T, Foyer CH, Parry MA, Forde BG, Araus JL, Hirel B, Schjoerring JK, Habash DZ (2008) Gene expression, cellular localisation and function of glutamine synthetase isozymes in wheat. Plant Mol Biol 67:89–105
Bourgis F, Roje S, Nuccio ML, Fisher DB, Tarczynski MC, Li C, Herschbach C, Rennenberg H, Pimenta MJ, Shen TL, Gage DA, Hanson AD (1999) S-Methylmethionine plays a major role in phloem sulfur transport and is synthesized by a novel type of methyltransferase. Plant Cell 11:1485–1498
Däschner K, Couée I, Binder S (2001) The mitochondrial isovaleryl-coenzyme a dehydrogenase of Arabidopsis oxidizes intermediates of leucine and valine catabolism. Plant Physiol 126:601–612
Elthon TE, Stewart CR (1981) Submitochondrial location and electron transport characteristics of enzymes involved in proline oxidation. Plant Physiol 67:780–784
Engell K (1989) Embryology of barley: time course and analysis of controlled fertilization and early embryo formation based on serial sections. Nord J Bot 9:265–280
Fait A, Hanhineva K, Beleggia R, Dai N, Rogachev I, Nikiforova VJ, Fernie AR, Aharoni A (2008) Reconfiguration of the chene and receptacle metabolic networks during strawberry fruit development. Plant Physiol 148:730–750
Goyer A, Collakova E, Shachar-Hill Y, Hanson AD (2007) Functional characterization of a methionine gamma-lyase in Arabidopsis and its implication in an alternative to the reverse trans-sulfuration pathway. Plant Cell Physiol 48:232–242
Hagel JM, Weljie AM, Vogel HJ, Facchini PJ (2008) Quantitative H-1 nuclear magnetic resonance metabolite profiling as a functional genomics platform to investigate alkaloid biosynthesis in opium poppy. Plant Physiol 147:1805–1821
Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR (2006) Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc 1:387–396
Miflin BJ, Habash DZ (2002) The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J Exp Bot 53:979–987
Miflin BJ, Lea PJ (1977) Amino acid metabolism. Annu Rev Plant Physiol 28:299–329
Moco S, Bino RJ, Vorst O, Verhoeven HA, de Groot J, van Beek TA, Vervoort J, De Vos RCH (2006) A liquid chromatography–mass spectrometry-based metabolome database for tomato. Plant Physiol 141:1205–1218
Moco S, Forshed J, De Vos RCH, Bino RJ, Vervoort J (2008) Intra- and inter-metabolite correlation spectroscopy of tomato metabolomics data obtained by liquid chromatography–mass-spectroscopy and nuclear magnetic resonance. Metabolomics 4:202–215
Mudd SH, Datko AH (1990) The S-methylmethionine cycle in Lemna paucicostata. Plant Physiol 93:623–630
Nelson T, Gandotra N, Tausta SL (2008) Plant cell types: reporting and sampling with new technologies. Curr Opin Plant Biol 11:567–573
Norstog K (1974) Nucellus during early embryogeny in barley: fine structure. Bot Gaz 135:97–103
Olsen O-A (2004) Nuclear endosperm development in cereals and Arabidopsis thaliana. Plant Cell 16:S214–S227
Ouyang J, Shao X, Li J (2000) Indole-3-glycerol phosphate, a branchpoint of indole-3-acetic acid biosynthesis from the tryptophan biosynthetic pathway in Arabidopsis thaliana. Plant J 24:327–333
Ranocha P, McNeil SD, Ziemak MJ, Li C, Tarczynski MC, Hanson AD (2001) The S-methylmethionine cycle in angiosperms: ubiquity, antiquity and activity. Plant J 25:575–584
Rébeillé F, Jabrin S, Bligny R, Loizeau K, Gambonnet B, Van Wilder V, Douce R, Ravanel S (2006) Methionine catabolism in Arabidopsis cells is initiated by a gamma-cleavage process and leads to S-methylcysteine and isoleucine syntheses. Proc Natl Acad Sci USA 103:15687–15692
Ricoult C, Echeverria LO, Cliquet JB, Limami AM (2006) Characterization of alanine aminotransferase (AlaAT) multigene family and hypoxic response in young seedlings of the model legume Medicago truncatula. J Exp Bot: 57:3079–3089
Rochat C, Wuilleme S, Boutin JP, Hedley CL (1995) A mutation at the rb gene, lowering ADPGPPase activity, affects storage product metabolism of pea seed coats. J Exp Bot 46:415–421
Schad M, Mungur R, Fiehn O, Kehr J (2005) Metabolic profiling of laser microdissected vascular bundles of Arabidopsis thaliana. Plant Methods 1:2
Schneider B, Hölscher D (2007) Laser microdissection and cryogenic nuclear magnetic resonance spectroscopy: an alliance for cell-type specific metabolite profiling. Planta 225:763–770
Seebauer JR, Moose SP, Fabbri BJ, Crossland LD, Below FE (2004) Amino acid metabolism in maize earshoots implications for assimilate preconditioning and nitrogen signaling. Plant Physiol 136:4326–4334
Sreenivasulu N, Altschmied L, Radchuk V, Gubatz S, Wobus U, Weschke W (2004) Transcript profiles and deduced changes of metabolic pathways in maternal and filial tissues of developing barley grains. Plant J 37:539–553
Thiel J, Weier D, Sreenivasulu N, Strickert M, Weichert N, Melzer M, Czauderna T, Wobus U, Weber H, Weschke W (2008) Different hormonal regulation of cellular differentiation and function in nucellar projection and endosperm transfer cells—a microdissection-based transcriptome study of young barley grains. Plant Physiol 148:1436–1452
Ward JL, Harris C, Lewis J, Beale MH (2003) Assessment of H-1 NMR spectroscopy and multivariate analysis as a technique for metabolite fingerprinting of Arabidopsis thaliana. Phytochemistry 62:949–957
Weber H, Borisjuk L, Wobus U (2005) Molecular physiology of legume seed development. Annu Rev Plant Biol 56:253–279
Weigelt K, Küster H, Radchuk R, Müller M, Weichert H, Fait A, Fernie AR, Saalbach I, Weber H (2008) Increasing amino acid supply in pea embryos reveals specific interactions of N and C metabolism and highlights the importance of mitochondrial metabolism. Plant J 55:909–926
Weschke W, Panitz R, Sauer N, Wang Q, Neubohn B, Weber H, Wobus U (2000) Sucrose transport into barley seeds: molecular characterisation of two transporters and implications for seed development and starch accumulation. Plant J 21:455–467
Weschke W, Panitz R, Gubatz S, Wang Q, Radchuk R, Weber H, Wobus U (2003) The role of invertases and hexose transporters in controlling sugar ratios in maternal and filial tissues of barley caryopses during early development. Plant J 33:395–411
Acknowledgments
We are grateful to Uta Siebert for her excellent and expert assistance in tissue processing and operating the PALM Laser Microbeam instrument. We also wish to thank Ursula Tiemann for graphical artwork. This work was supported by the Deutsche Forschungsgemeinschaft (DFG, FKZ 39205123) and by the Federal Ministry of Education and Research (BMBF, FKZ 0313821A).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thiel, J., Müller, M., Weschke, W. et al. Amino acid metabolism at the maternal–filial boundary of young barley seeds: a microdissection-based study. Planta 230, 205–213 (2009). https://doi.org/10.1007/s00425-009-0935-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-009-0935-1