Abstract
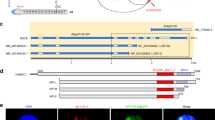
In plants, there are no DNA polymerase β (Pol β) and DNA ligase III (Lig3) genes. Thus, the plant short-patch base excision repair (short-patch BER) pathway must differ considerably from that in mammals. We characterized the rice (Oryza Sativa L. cv. Nipponbare) homologue of the mammalian X-ray repair cross complementing 1 (XRCC1), a well-known BER protein. The plant XRCC1 lacks the N-terminal domain (NTD) which is required for Pol β binding and is essential for mammalian cell survival. The recombinant rice XRCC1 (OsXRCC1) protein binds single-stranded DNA (ssDNA) as well as double-stranded DNA (dsDNA) and also interacts with rice proliferating cell nuclear antigen (OsPCNA) in a pull-down assay. Through immunoprecipitation, we demonstrated that OsXRCC1 forms a complex with PCNA in vivo. OsXRCC1 mRNA was expressed in all rice organs and was induced by application of bleomycin, but not of MMS, H2O2 or UV-B. Bleomycin also increased the fraction of OsXRCC1 associated with chromatin. These results suggest that OsXRCC1 contributes to DNA repair pathways that differ from the mammalian BER system.





Similar content being viewed by others
Abbreviations
- AP sites:
-
Apurinic/apyrimidinic sites
- GFP:
-
Green fluorescent protein
- GST:
-
Glutathione S-transferase
- LpBER:
-
Long-patch base excision repair
- MMS:
-
Methylmethanesulfonate
- SAM:
-
Shoot apical meristem
- SpBER:
-
Short-patch base excision repair
- XRCC1:
-
X-ray repair cross complementing 1
References
Audebert M, Salles B, Calsou P (2004) Involvement of poly(ADP-ribose) polymerase-1 and XRCC1/DNA ligase III in an alternative route for DNA double-strand breaks rejoining. J Biol Chem 279:55117–55126
Balajee AS, Dianova I, Bohr VA (1999) Oxidative damage-induced PCNA complex formation is efficient in xeroderma pigmentosum group A but reduced in Cockayne syndrome group B cells. Nucleic Acids Res 27:4476–4482
Brem R, Hall J (2005) XRCC1 is required for DNA single-strand break repair in human cells. Nucleic Acids Res 33:2512–2520
Caldecott KW, McKeown CK, Tucker JD, Ljungquist S, Thompson LH (1994) An interaction between the mammalian DNA repair protein XRCC1 and DNA ligase III. Mol Cell Biol 14:68–76
Caldecott KW, Tucker JD, Stanker LH, Thompson LH (1995) Characterization of the XRCC1-DNA ligase III complex in vitro and its absence from mutant hamster cells. Nucleic Acids Res 23:4836–4843
Caldecott KW, Aoufouchi S, Johnson P, Shall S (1996) XRCC1 polypeptide interacts with DNA polymerase β and possibly poly (ADP-ribose) polymerase, and DNA ligase III is a novel molecular ‘nick-sensor’ in vitro. Nucleic Acids Res 24:4387–4394
Caldecott KW (2003) XRCC1 and strand break repair. DNA Repair (Amst) 2:955–969
Cappelli E, Taylor R, Cevasco M, Abbondandolo A, Caldecott K, Frosina G (1997) Involvement of XRCC1 and DNA ligase III gene products in DNA base excision repair. J Biol Chem 272:23970–23975
Chiu W, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J (1996) Engineered GFP as a vital reporter in plants. Curr Biol 6:325–330
Dianova II, Sleeth KM, Allinson SL, Parsons JL, Breslin C, Caldecott KW, Dianov GL (2004) XRCC1-DNA polymerase β interaction is required for efficient base excision repair. Nucleic Acids Res 32:2550–2555
Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H (2002) Free radical-induced damage to DNA: mechanisms and measurement. Free Radic Biol Med 32:1102–1115
Dogliotti E, Fortini P, Pascucci B, Parlanti E (2001) The mechanism of switching among multiple BER pathways. Prog Nucleic Acid Res Mol Biol 68:3–27
Fan J, Otterlei M, Wong HK, Tomkinson AE, Wilson DM 3rd (2004) XRCC1 co-localizes and physically interacts with PCNA. Nucleic Acids Res 32:2193–2201
Fortini P, Dogliotti E (2007) Base damage and single-strand break repair: mechanisms and functional significance of short- and long-patch repair subpathways. DNA Repair 6:398–409
Griffiths D, Uchiyama M, Nurse P, Wang TS (2000) A novel mutant allele of the chromatin-bound fission yeast checkpoint protein Rad17 separates the DNA structure checkpoints. J Cell Sci 113:1075–1088
Kimura S, Tahira Y, Ishibashi T, Mori Y, Mori T, Hashimoto J, Sakaguchi K (2004) DNA repair in higher plants; photoreactivation is the major DNA repair pathway in non-proliferating cells while excision repair (nucleotide excision repair and base excision repair) is active in proliferating cells. Nucleic Acids Res 32:2760–2767
Kimura S, Sakaguchi K (2006) DNA repair in plants. Chem Rev 106:753–766
Krokan HE, Standal R, Slupphaug G (1997) DNA glycosylases in the base excision repair of DNA. Biochem J 325:1–16
Leppard JB, Dong Z, Mackey ZB, Tomkinson AE (2003) Physical and functional interaction between DNA ligase IIIα and poly(ADP-Ribose) polymerase 1 in DNA single-strand break repair. Mol Cell Biol 23:5919–5927
Maher RL, Bloom LB (2007) Pre-steady-state kinetic characterization of the AP endonuclease activity of human AP endonuclease 1. J Biol Chem 282:30577–30585
Marintchev A, Mullen MA, Maciejewski MW, Pan B, Gryk MR, Mullen GP (1999) Solution structure of the single-strand break repair protein XRCC1 N-terminal domain. Nat Struct Biol 6:884–893
Masson M, Niedergang C, Schreiber V, Muller S, Menissier-de Murcia J, de Murcia G (1998) XRCC1 is specifically associated with poly(ADP-ribose) polymerase and negatively regulates its activity following DNA damage. Mol Cell Biol 18:3563–3571
Nakamura J, Swenberg JA (1999) Endogenous apurinic/apyrimidinic sites in genomic DNA of mammalian tissues. Cancer Res 59:2522–2526
Namekawa S, Hamada F, Sawado T, Ishii S, Nara T, Ishizaki T, Ohuchi T, Arai T, Sakaguchi K (2003) Dissociation of DNA polymerase α-primase complex during meiosis in Coprinus cinereus. Eur J Biochem 270:2137–2146
Parlanti E, Locatelli G, Maga G, Dogliotti E (2007) Human base excision repair complex is physically associated to DNA replication and cell cycle regulatory proteins. Nucleic Acids Res 35:1569–1577
Parsons JL, Dianova II, Allinson SL, Dianov GL (2005) DNA polymerase β promotes recruitment of DNA ligase III α-XRCC1 to sites of base excision repair. Biochemistry 44:10613–10619
Puebla-Osorio N, Lacey DB, Alt FW, Zhu C (2006) Early embryonic lethality due to targeted inactivation of DNA ligase III. Mol Cell Biol 26:3935–3941
Shibata Y, Nakamura T (2002) Defective flap endonuclease 1 activity in mammalian cells is associated with impaired DNA repair and prolonged S phase delay. J Biol Chem 277:746–754
Sugo N, Aratani Y, Nagashima Y, Kubota Y, Koyama H (2000) Neonatal lethality with abnormal neurogenesis in mice deficient in DNA polymerase β. EMBO J 19:1397–1404
Taylor RM, Hamer MJ, Rosamond J, Bray CM (1998) Molecular cloning and functional analysis of the Arabidopsis thaliana DNA ligase I homologue. Plant J 14:75–81
Taylor RM, Thistlethwaite A, Caldecott KW (2002) Central role for the XRCC1 BRCT I domain in mammalian DNA single-strand break repair. Mol Cell Biol 22:2556–2563
Tebbs RS, Flannery ML, Meneses JJ, Hartmann A, Tucker JD, Thompson LH, Cleaver JE, Pedersen RA (1999) Requirement for the Xrcc1 DNA base excision repair gene during early mouse development. Dev Biol 208:513–529
Thompson LH, Brookman KW, Dillehay LE, Carrano AV, Mazrimas JA, Mooney CL, Minkler JL (1982) A CHO-cell strain having hypersensitivity to mutagens, a defect in DNA strand-break repair, and an extraordinary baseline frequency of sister-chromatid exchange. Mutat Res 95:427–440
Toschi L, Bravo R (1988) Changes in cyclin/proliferating cell nuclear antigen distribution during DNA repair synthesis. J Cell Biol 107:1623–1628
Uchiyama Y, Kimura S, Yamamoto T, Ishibashi T, Sakaguchi K (2004) Plant DNA polymerase λ, a DNA repair enzyme that functions in plant meristematic and meiotic tissues. Eur J Biochem 271:2799–2807
West CE, Waterworth WM, Jiang Q, Bray CM (2000) Arabidopsis DNA ligase IV is induced by gamma-irradiation and interacts with an Arabidopsis homologue of the double strand break repair protein XRCC4. Plant J 24:67–78
Acknowledgments
We express our appreciation to Dr. Y. Niwa (Laboratory of Plant Cell Technology, Graduate School of Nutritional and Environmental Sciences, University of Shizuoka) for providing the CaMV35S-sGFP(S65T)-nos3′ SK vector. Y. U. was supported by a Research Fellowships of the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uchiyama, Y., Suzuki, Y. & Sakaguchi, K. Characterization of plant XRCC1 and its interaction with proliferating cell nuclear antigen. Planta 227, 1233–1241 (2008). https://doi.org/10.1007/s00425-008-0695-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-008-0695-3