Abstract
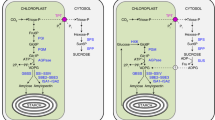
The presence of sucrose (Suc) in plastids was questioned for several decades. Although it was reported some decades ago, neither Suc transporters nor Suc metabolizing enzymes were demonstrated to be active in those organelles. By biochemical, immunological, molecular and genetic approaches we show that alkaline/neutral invertases (A/N-Invs) are also localized in chloroplasts of spinach and Arabidopsis. A/N-Inv activity and polypeptide content were shown in protein extracts from intact chloroplasts. Moreover, we functionally characterized the Arabidopsis At-A/N-InvE gene coding for a chloroplast-targeted A/N-Inv. The At-A/N-InvE knockout plants displayed a lower total A/N-Inv activity in comparison with wild-type plants. Furthermore, neither A/N-Inv activity nor A/N-Inv polypeptides were detected in protein extracts prepared from chloroplasts of mutant plants. Also, the measurement of carbohydrate content, in leaves harvested either at the end of the day or at the end of the night period, revealed that the knockout plants showed a decrease in starch accumulation but no alteration in Suc levels. These are the first results demonstrating the presence of a functional A/N-Inv inside chloroplasts and its relation with carbon storage in Arabidopsis leaves. Taken together our data and recent reports, we conclude that the participation of A/N-Invs in the carbon flux between the cytosol and the plastids may be a general phenomenon in plants.





Similar content being viewed by others
Abbreviations
- A/N-Inv:
-
Alkaline/neutral invertase
- Chl:
-
Chlorophyll
- Col 0:
-
Arabidopsis thaliana ecotype Columbia 0
- Inv:
-
Invertase
- gfp:
-
Green fluorescent protein
- Suc:
-
Sucrose
- UDP-Glc:
-
UDP-glucose
- UGPase:
-
UDP-glucose pyrophosphorylase
References
Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, Gadrinab C, Heller C, Jeske A, Koesema E, Meyers CC, Parker H, Prednis L, Ansari Y, Choy N, Deen H, Geralt M, Hazari N, E Hom, Karnes M, Mulholland C, Ndubaku R, Schmidt I, Guzman P, Aguilar-Henonin L, Schmid M, Weigel D, Carter DE, Marchand T, Risseeuw E, Brogden D, Zeko A, Crosby WL, Berry CC, Ecker JR (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:653–657
Altschul SF, Meadden T, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Amor Y, Haigler CH, Johnson S, Wainscott M, Delmer DP (1995) A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Poc Natl Acad Sci USA 92:9353–9357
Asatsuma S, Sawada C, Itoh K, Okito M, Kitajima A, Mitsui T (2005) Involvement of α-amylase I-1 in starch degradation in rice chloroplasts. Plant Cell Physiol 46:858–869
Ashwell G (1957) Colorimetric analysis of sugars. In: Colowik SP, Kaplan NO (eds) Methods enzymol, vol 3. Academic, New York, pp 73–105
Beers EP, Moreno TN, Callis J (1992) Subcellular localization of ubiquitin and ubiquitinated proteins in Arabidopsis thaliana. J Biol Chem 267:15432–15439
Bergmeyer HU, Bernt E, Schmidt F, Stork H (1974) d-Glucose: determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 3. Academic, New York, pp 1196–1201
Bernt E, Bergmeyer HU (1974) d-Fructose. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 3. Academic, New York, pp 1304–1307
Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Görlach J (2001) Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13:1499–1510
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 7:248–254
Chen JQ, Black CC (1992) Biochemical and immunological properties of alkaline invertase isolated from sprouting soybean hypocotyls. Arch Biochem Biophys 295:61–69
Chen M-H, Huang L-F, Li H-M, Chen Y-R, Yu S-M (2004) Signal peptide-dependent targeting of a rice α-amylase and cargo proteins to plastids and extracellular compartments of plant cells. Plant Physiol 135:1367–1377
Cho Y-H, Yoo S-D, Sheen J (2006) Regulatory functions of nuclear hexokinase1 complex in glucose signaling. Cell 127:579–589
Cooper TG, Beevers H (1969) Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constituents and catalytic capacity. J Biol Chem 244:3507–3513
Cormack BP, Valdivia RH, Falkow S (1996) FACS-optimized mutants of the green fluorscent protein (gfp). Gene 173:33–38
Dennis DT, Blakeley SD (2000) Carbohydrate metabolism. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, pp 630–675
Edelman J, Schoolar AI, Bonnor HW (1971) Permeability of sugar-cane chloroplasts to sucrose. J Exp Bot 22:534–545
Felsenstein J (1993) PHYLIP (Phylogeny inference package), version 3.5c. University of Washington, Seattle
Fukushima E, Arata Y, Endo T, Sonnewald U, Sato F (2001) Improved salt tolerance of transgenic tobacco expressing apoplastic yeast-derived invertase. Plant Cell Physiol 42:245–249
Gallagher JA, Pollock CJ (1998) Isolation and characterization of a cDNA clone from Lolium temulentum encoding for a Suc hydrolytic enzyme which shows alkaline/neutral invertase activity. J Exp Bot 49:789–795
Gerrits N, Turk SCHJ, van Dun KPM, Hulleman SHD, Visser RGF, Weisbeek PJ, Smeekens SCM (2001) Sucrose metabolism in plastids. Plant Physiol 125:926–934
Giese JO, Herbers K, Hoffmann M, Klösgen RB, Sonnewald U (2005) Isolation and functional characterization of a novel plastidic hexokinase from Nicotiana tabacum. FEBS Lett 579:827–831
Hauser M, Eichelman H, Oja V, Heber U, Laik A (1995) Stimulation by light of rapid pH regulation in the chloroplast stroma in vivo as indicated by CO2 solubilization in leaves. Plant Physiol 108:1059–1066
Heineke D, Wildenberger K, Sonnewald U, Willmitzer L, Heldt HW (1994) Accumulation of hexoses in leaf vacuoles: studies with transgenic tobacco plants expressing yeast-derived invertase in the cytosol, vacuole or apoplasm. Planta 194:29–33
Heldt HW, Sauer F (1971) The inner membrane of the chloroplast envelope as site of specific metabolite transport. Biochim Biophys Acta 234:83–91
Hendriks JH, Kolbe A, Gibon Y, Stitt M, Geigenberger P (2003) ADP-glucose pyrophosphorylase is activated by posttranslational redox-modification in response to light and to sugars in leaves of Arabidopsis and other plant species. Plant Physiol 133:838–849
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil, Univ Calif Agric Exp Stn, Berkeley, CA, Circular No 347, pp 1–39
Huber SC, Huber JL (1996) Role and regulation of sucrose-phosphate synthase in higher plants. Annu Rev Plant Physiol Plant Mol Biol 47:431–444
Ji X, Van den Ende W, Van Laere A, Cheng S, Bennett J (2005) Structure, evolution, and expression of the two invertase gene families of rice. J Mol Evol 60:615–634
Jones MGK, Outlaw WH, Lowry OH (1977) Enzymatic assay of 10−7 to 10−4 moles of Suc in plant tissue. Plant Physiol 60:379–383
Kessler F, Schnell DJ (2006) The function and diversity of plastid protein import pathways: a multilane GTPase highway into plastids. Traffic 7:248–257
Koch K (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7:235–246
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee DW, Lee S, Lee G-j, Lee KH, Kim S, Cheong GW, Hwang I (2006) Functional characterization of sequence motifs in the transit peptide of Arabidopsis small subunit of rubisco. Plant Physiol 140:466–483
Lee HS, Sturm A (1996) Purification and characterization of neutral and alkaline invertase from carrot. Plant Physiol 112:1513–1522
López-Juez E (2007) Plastid biogenesis, between light and shadows. J Exp Bot 58:11–26
López-Juez E, Pyke KA (2005) Plastids unleashed: their development and their integration in plant development. Int J Dev Biol 49:557–577
Lou Y, Gou J-Y, Xue H-W (2007) PIP5K9, an Arabidopsis phosphatidylinositol monophosphate kinase, interacts with a cytosolic invertase to negatively regulate sugar-mediated root growth. Plant Cell 19:163–181
Lunn J, Ashton A, Hatch M, Heldt H (2000) Purification, molecular cloning, and sequence analysis of Suc-6-phosphate phosphohydrolase from plants. Proc Natl Acad Sci USA 97:12914–12919
Mackinney G (1941) Absortion of light by chlorophyll solutions. J Biol Chem 140:315–322
Martin C, Smith AM (1995) Starch biosynthesis. Plant Cell 7:971–985
Mathur J, Koncz C (1998) Protoplasts isolation, culture and regeneration. In: Martínez-Zapater JM, Salinas J (eds) Arabidopsis protocols. Methods in molecular biology, vol 8. Humana Press, Totowa, NJ, pp 35–42
Mourioux G, Douce R (1981) Slow passive diffusion of orthophosphate between intact isolated chloroplasts and suspending medium. Plant Physiol 67:470–473
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–497
Murayama H, Handa S (2007) Genes for alkaline/neutral invertase in rice: alkaline/neutral invertases are located in plant mitochondria and also in plastids. Planta 225:1193–1203
Paul MJ, Foyer CH (2001) Sink regulation of photosynthesis. J Exp Bot 52:1383–1400
Porchia AC, Fiol DF, Salerno GL (1999) Differential synthesis of sucrose and trehalose in Euglena gracilis cells during growth and salt stress. Plant Sci 149:43–49
Renart J, Sandoval IV (1984) Western blots. In: Jacoby WB (eds) Methods enzymol, vol 104, Academic, Orlando, pp 455–460
Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell 14(Suppl):S185–S205
Ross HA, McRae D, Davies HV (1996) Sucrolytic enzyme activities in cotyledons of the faba bean (developmental changes and purification of alkaline invertase). Plant Physiol 111:329–338
Rujan T, Martin W (2001) How many genes in Arabidopsis come from cyanobacteria? An estimate from 386 protein phylogenies. Trends Genet 17:113–120
Salerno GL, Pagnussat GC, Pontis HG (1998) Studies on Suc-phosphate synthase from rice leaves. Cell Mol Biol 44:404–416
Salerno G, Curatti L (2003) Origin of sucrose metabolism in higher plants: when, how and why? Trends Plant Sci 8:63–69
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Santarius KA, Milde H (1977) Sugar compartmentation in frost-hardy and partially deharded cabbage leaf cells. Planta 136:163–166
Scheibe R, Stitt M (1988) Comparison of NADP-malate dehydrogenase activation, QA reduction and O2 evolution in spinach leaves. Plant Physiol Biochem 26:473–481
Sharkey TD, Laporte M, Lu Y, Weise S, Weber APM (2004) Engineering plants for elevated CO2: a relationship between starch degradation and sugar sensing. Plant Biol 6:280–288
Sheen J, Zhou L, Jang JC (1999) Sugars as signaling molecules. Curr Opin Plant Biol 2:410–718
Smith AM, Zeeman SC, Smith SM (2005) Starch degradation. Annu Rev Plant Biol 56:73–98
Sowokinos J (1981) Pyrophosphorylases in Solanum tuberosum. Plant Physiol 68:924–929
Stitt M, Heldt HW (1981) Physiological rates of starch breakdown in isolated intact spinach chloroplasts. Plant Physiol 68:755–761
Sturm A (1999) Invertases. Primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol 121:1–7
Sturm A, Hess D, Lee HS, Lienhard S (1999) Neutral invertase is a novel type of Suc-cleaving enzyme. Physiol Plant 107:159–165
Subbaiah CC, Palaniappan A, Duncan K, Rhoads DM, Huber SC, Sachs MM (2006) Mitochondrial localization and putative signaling function of sucrose synthase in maize. J Biol Chem 281:15625–15635
Tetlow IJ, Morell MK, Emes MJ (2004) Recent developments in understanding the regulation of starch metabolism in higher plants. J Exp Bot 55:2131–2145
Thompson JD, Gibson TJ, Plewniak FM, Jeanmougin F, Higgins DG (1997) The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 15:4876–4882
Tiessen A, Hendriks JHM, Stitt M, Branscheid A, Gibon Y, Farré EM, Geigenberger P (2002) Starch synthesis in potato tubers is regulated by post-translational redox modification of ADP-glucose pyrophosphorylase: a novel regulatory mechanism linking starch synthesis to the sucrose supply. Plant Cell 14:2191:2213
Van den Ende W, Van Laere A (1995) Purification and properties of a neutral invertase from the roots of Cichorium intybus. Physiol Plant 93:241–248
Vargas W, Cumino AC, Salerno GL (2003) Cyanobacterial alkaline/neutral invertases. Origin of Suc hydrolysis in the plant cytosol? Planta 216:951–960
Vargas WA, Pontis HG, Salerno GL (2007) Differential expression of alkaline and neutral invertases in response to environmental stresses: characterization of an alkaline isoform as a stress-response enzyme in wheat leaves. Planta 226:1535–1545
Vorster DJ, Botha FC (1998) Partial purification and characterization of sugarcane neutral invertase. Phytochemistry 49:651–655
Waegemann K, Soll J (1996) Phosphorylation of the transit sequence of chloroplast precursor proteins. J Biol Chem 271:6545–6554
Walker DA (1974) Chloroplast and cell. The movement of certain key substances, etc. across the chloroplast envelope. In: Northcote DH (ed) MTP International review of science, biochemistry series one, vol 11. Butterworth, London, pp 1–49
Walker RP, Winters AL, Pollock CJ (1997) Purification and characterization of invertases from leaves of Lolium temulentum. New Phytol 135:259–266
Wang CT, Nobel PS (1971) Permeability of pea chloroplastst alcohols and aldoses as measured by reflection coefficients. Biochim Biophys Acta 241:200–212
Winter H, Huber SC (2000) Regulation of sucrose metabolism in higher plants: localization and regulation of activity of key enzymes. Crit Rev Plant Sci 19:31–67
Winter H, Robinson DG, Heldt HW (1994) Subcellular volumes and metabolite concentrations in spinach leaves. Planta 193:530–535
Wroblewski T, Tomczak A, Michelmore R (2005) Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis. Plant Biotech J 3:259–273
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
425_2007_657_MOESM1_ESM.tif
Fig. S1 Phylogenetic analysis of A/N-Invs. Unrooted neighbour-joining phylograms were constructed after sequence alignments of plant and cyanobacterial A/N-Inv sequences with the CLUSTAL X program using BLOSSUM matrix and a bootstrap trial of 1,000. Qualitatively similar tree topologies were observed when using maximum parsimony algorithm. The graphical representation was generated using the Treeview16 program.(TIF 476 kb)
Rights and permissions
About this article
Cite this article
Vargas, W.A., Pontis, H.G. & Salerno, G.L. New insights on sucrose metabolism: evidence for an active A/N-Inv in chloroplasts uncovers a novel component of the intracellular carbon trafficking. Planta 227, 795–807 (2008). https://doi.org/10.1007/s00425-007-0657-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-007-0657-1