Abstract
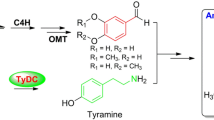
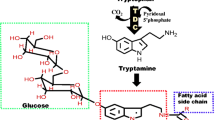
l-Tryptophan decarboxylase (TDC) and l-tyrosine decarboxylase (TYDC) belong to a family of aromatic l-amino acid decarboxylases and catalyze the conversion of tryptophan and tyrosine into tryptamine and tyramine, respectively. The rice genome has been shown to contain seven TDC or TYDC-like genes. Three of these genes for which cDNA clones were available were characterized to assign their functions using heterologous expression in Escherichia coli and rice (Oryza sativa cv. Dongjin). The purified products of two of the genes were expressed in E. coli and exhibited TDC activity, whereas the remaining gene could not be expressed in E. coli. The recombinant TDC protein with the greatest TDC activity showed a K m of 0.69 mM for tryptophan, and its activity was not inhibited by phenylalanine or tyrosine, indicating a high level of substrate specificity toward tryptophan. The ectopic expression of the three cDNA clones in rice led to the abundant production of the products of the encoded enzymes, tyramine and tryptamine. The overproduction of TYDC resulted in stunted growth and a lack of seed production due to tyramine accumulation, which increased as the plant aged. In contrast, transgenic plants that produced TDC showed a normal phenotype and contained 25-fold and 11-fold higher serotonin in the leaves and seeds, respectively, than the wild-type plants. The overproduction of either tyramine or serotonin was not strongly related to the enhanced synthesis of tyramine or serotonin derivatives, such as feruloyltyramine and feruloylserotonin, which are secondary metabolites that act as phytoalexins in plants.








Similar content being viewed by others
Abbreviations
- TDC:
-
l-Tryptophan decarboxylase
- TYDC:
-
l-Tyrosine decarboxylase
- HPLC:
-
High performance liquid chromatography
- AADCs:
-
Aromatic l-amino acid decarboxylases
References
Back K, Yin S, Chappell J (1994) Expression of a plant sesquiterpene cyclase gene in Escherichia coli. Arch Biochem Biophys 315:527–532
Berlin J, Rugenhagen C, Dietze P, Fecker LF, Goddijn OJM, Hoge JHC (1993) Increased production of serotonin by suspension and root cultures of Peganum harmala transformed with a tryptophan decarboxylase cDNA clone from Catharanthus roseus. Transgenic Res 2:336–344
Christou P, Barton KA (1989) Cytokinin antagonist activity of substituted phenethylamines in plant cell culture. Plant Physiol 89:564–568
Clarke DD (1982) The accumulation of cinnamic acid amides in the cell walls of potato tissue as an early response to fungal attacks. In: Wood RKS (ed) Active defense mechanisms in plants. Plenum, New York, pp 321–332
De Luca V, Marineau C, Brisson N (1989) Molecular cloning and analysis of cDNA encoding a plant tryptophan decarboxylase: comparison with animal dopa decarboxylase. Proc Natl Acad Sci USA 86:2582–2586
Facchini PJ, De Luca V (1994) Differential and tissue-specific expression of a gene family for tyrosine/dopa decarboxylase in opium poppy. J Biol Chem 269:26684–26690
Facchini PJ, Yu M, Penzes-Yost C (1999) Decreased cell wall digestibility in canola transformed with chimeric tyrosine decarboxylase genes from opium poppy. Plant Physiol 120:653–664
Facchini PJ, Huber-Allanach KL, Tari LW (2000) Plant aromatic l-amino acid decarboxylases: evolution, biochemistry, regulation, and metabolic engineering applications. Phytochemistry 54:121–138
Fiore SD, Li Q, Leech MJ, Schuster F, Emans N, Fischer R, Schillberg S (2002) Targeting tryptophan decarboxylase to selected subcellular compartments of tobacco plants affects enzyme stability and in vivo function and leads to a lesion-mimic phenotype. Plant Physiol 129:1160–1169
Furutani I, Sukegawa S, Kyozuka J (2006) Genome-wide analysis of spatial and temporal gene expression in rice panicle development. Plant J 46:503–511
Gibson RA, Barret G, Wightman F (1972) Biosynthesis and metabolism of indole-3yl-acetic acid. III. Partial purification and properties of a tryptamine-forming l-tryptophan decarboxylase from tomato shoots. J Exp Bot 23:775–786
Grandmaison J, Olah GM, Van Calsteren MR, Furlan V (1993) Characterization and localization of plant phenolics likely involved in the pathogen resistance expressed by endomycorrhizal roots. Mycorrhiza 3:155–164
Guillet G, De Luca V (2005) Wound-inducible biosynthesis of phytoalexin hydroxycinnamic acid amides of tyramine in tryptophan and tyrosine decarboxylase transgenic tobacco lines. Plant Physiol 137:692–699
Guillet G, Poupart J, Basurco J, De Luca V (2000) Expression of tryptophan decarboxylase and tyrosine decarboxylase genes in tobacco results in altered biochemical and physiological phenotypes. Plant Physiol 122:933–943
Jang SM, Ishihara A, Back K (2004) Production of coumaroylserotonin and feruloylserotonin in transgenic rice expressing pepper hydroxycinnamoyl-coenzyme A: serotonin N-(hydroxycinnamoyl)transferase. Plant Physiol 135:346–356
Kaminaga Y, Schnepp J, Peel G, Kish CM, Ben-Nissan G, Weiss D, Orlova I, Lavie O, Rhodes D, Wood K, Porterfield DM, Cooper AJL, Schloss JV, Pichersky E, Vainstein A, Dudareva N (2006) Plant phenylacetaldehyde synthase is a bifunctional homotetrameric enzyme that catalyzes phenylalanine decarboxylase and oxidation. J Biol Chem 281:23357–23366
Kang K, Jang SM, Kang S, Back K (2005) Enhanced neutraceutical serotonin derivatives of rice by hydroxycinnamoyl-CoA: serotonin N-(hydroxycinnamoyl)transferase. Plant Sci 168:783–788
Kang S, Kang K, Chung GC, Choi D, Ishihara A, Lee DS, Back K (2006) Functional analysis of the amine substrate specificity domain of pepper tyramine and serotonin N-hydroxycinnamoyltransferases. Plant Physiol 140:704–715
Kang S, Kang K, Lee K, Back K (2007) Characterization of tryptamine 5-hydroxylase and serotonin synthesis in rice plants. Plant Cell Rep DOI 10.1007/s00299-007-0405-9
Kawalleck P, Keller H, Hahlbrock K, Scheel D, Somssich IE (1993) A pathogen-responsive gene of parsley encodes tyrosine decarboxylase. J Biol Chem 268:2189–2194
Lee HJ, Lee SB, Chung JS, Han SU, Han O, Guh JO, Jeon JS, An G, Back K (2000) Transgenic rice plants expressing a Bacillus subtilis protoporphyrinogen oxidase gene are resistant to diphenyl ether herbicide oxyfluorfen. Plant Cell Physiol 41:743–749
Lee DE, Kang K, Lee SG, Back K (2007) Enhanced synthesis of feruloyltyramine and 4-coumaroyltyramine is associated with tyramine availability in transgenic rice expressing pepper tyramine N-hydroxycinnamoyltransferase. Plant Sci 172:57–63
López-Meyse M, Nessler CL (1997) Tryptophan decarboxylase is encoded by two autonomously regulated genes in Camptotheca acuminate which are differentially expressed during development and stress. Plant J 11:1167–1175
Mathis JR, Back K, Starks C, Noel J, Poulter CD, Chappell J (1997) Pre-steady-state study of recombinant sesquiterpene cyclases. Biochemistry 36:8340–8348
Matsuda F, Miyagawa H, Ueno T (2000) β-1,3-Glucooligosaccharide induced activation of four enzymes responsible for N-p-coumaroyloctopamine biosynthesis in potato (Solanum tuberosum cv.) tuber tissue. Z Naturforsch 55c:373–382
Murch SJ, Campbell SSB, Saxena P (2001) The role of serotonin and melatonin in plant morphogenesis: regulation of auxin-induced root organogenesis in in vitro-cultured explants of St. John’s wort (Hypericum perforatum L.). In Vitro Cell Dev Biol Plant 37:786–793
Negrel J, Lherminier J (1987) Peroxidase-mediated integration of tyramine into xylem cell walls of tobacco leaves. Planta 172:494–501
Negrel J, Javelle F, Paynot M (1993) Biochemical basis of resistance of tobacco callus tissue cultures to hydroxyphenylethylamines. Plant Physiol 103:329–334
Noé W, Mollenschott C, Berlin J (1984) Tryptophan decarboxylase from Catharanthus roseus cell suspension cultures: purification, molecular and kinetic data of the homogenous protein. Plant Mol Biol 3:281–288
Odjakova M, Hadjiivanova C (1997) Animal neurotransmitter substances in plants. Bulg J Plant Physiol 23:94–102
Ohyanagi H, Tanaka T, Sakai H, Shigemoto Y, Yamaguchi K, Habara T, Fujii Y, Antonio BA, Nagamura Y, Imanishi T, Ikeo K, Itoh T, Gojobori T, Sasaki T (2006) The rice annotation project database (RAP-DB): hub for Oryza sativa spp. japonica genome information. Nucleic Acids Res 34:D741–D744
Qu LQ, Takaiwa F (2004) Evaluation of tissue specificity and expression strength of rice seed component gene promoters in transgenic rice. Plant Biotechnol J 2:113–125
Schröder P, Abele C, Gohr P, Stuhlfauth-Roisch U, Grosse W (1999) Latest on the enzymology of serotonin biosynthesis in walnut seeds. Adv Exp Med Biol 467:637–644
Tanaka E, Tanaka C, Mori N, Kuwahara Y, Tsuda M (2003) Phenylpropanoid amides of serotonin accumulate in witches’ broom diseased bamboo. Phytochemistry 64:965–969
Ueno M, Shibata H, Kihara J, Hnda Y, Arase S (2003) Increased tryptophan decarboxylase and monoamine oxidase activities induce Sekiguchi lesion formation in rice infected with Magnaporthe grisea. Plant J 36:215–228
Yamazaki Y, Sudo H, Yamazaki M, Aimi N, Saito K (2003) Camptothecin biosynthesis genes in hairy roots of Ophiorrhiza pumila: cloning, characterization and differential expression in tissues and by stress compounds. Plant Cell Physiol 44:395–403
Yao K, De Luca V, Brisson N (1995) Creation of a metabolic sink for tryptophan alters the phenylpropanoid pathway and the susceptibility of potato to Phytophthora infestans. Plant Cell 7:1787–1799
Yu J, Hu S, Wang J, Wong GK, Li S, Liu B, Deng Y, Dai L, Zhou Y, Zhang X, et al (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 296:79–92
Acknowledgments
We gratefully acknowledge Dr. Atsushi Ishihara for providing authentic feruloylserotonin and 4-coumaroylserotonin. This work was supported by the SRC program of MOST/KOSEF through the Agricultural Plant Stress Research Center (R11-2001-092-03001-0).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, S., Kang, K., Lee, K. et al. Characterization of rice tryptophan decarboxylases and their direct involvement in serotonin biosynthesis in transgenic rice. Planta 227, 263–272 (2007). https://doi.org/10.1007/s00425-007-0614-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-007-0614-z