Abstract
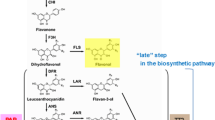
Anthocyanins are the largest group of plant pigments responsible for colors ranging from red to violet and blue. The biosynthesis of anthocyanins, as part of the larger phenylpropanoid pathway, has been characterized in great detail. In contrast to the detailed molecular knowledge available on anthocyanin synthesis, very little is known about the stability and catabolism of anthocyanins in plants. In this study we present a preliminary characterization of active in planta degradation of anthocyanins, requiring novel mRNA and protein synthesis, in Brunfelsia calycina flowers. Brunfelsia is a unique system for this study, since the decrease in pigment concentration in its flowers (from dark purple to white) is extreme and rapid, and occurs at a specific and well-defined stage of flower development. Treatment of detached flowers with protein and mRNA synthesis inhibitors, at specific stages of flower development, prevented degradation. In addition, treatment of detached flowers with cytokinins delayed senescence without changing the rate of anthocyanin degradation, suggesting that degradation of anthocyanins is not part of the general senescence process of the flowers but rather a distinctive and specific pathway. Based on studies on anthocyanin degradation in wine and juices, peroxidases are reasonable candidates for the in vivo degradation. A significant increase in peroxidase activity was shown to correlate in time with the rate of anthocyanin degradation. An additional indication that oxidative enzymes are involved in the process is the fact that treatment of flowers with reducing agents, such as DTT and glutathione, caused inhibition of degradation. This study represents the first step in the elucidation of the molecular mechanism behind in vivo anthocyanin degradation in plants.








Similar content being viewed by others
References
Andrews J, Malone M, Thompson DS, Ho LC, Burton KS (2000) Peroxidase isozyme patterns in the skin maturing tomato fruit. Plant Cell Environ 23:415–422
Bartoli CG, Simontacchi M, Guiamet JJ, Montaldi E, Puntarulo S (1995) Antioxidant enzymes and lipid-peroxidation during aging of Chrysanthemum-morifolium Ram petals. Plant Sci 104:161–168
Borochov A, Mayak S, Halevy AH (1976) Combined effects of abscisic acid and sucrose on growth and senescence of rose flowers. Physiol Plant 36:221–224
Bradley JM, Rains SR, Manson JL, Davies KM (2000) Flower pattern stability in genetically modified lisianthus (Eustoma grandiflorum) under commercial growing conditions. N Z J Crop Hortic Sci 28:175–184
Brenzel KN (2001) Western Garden Book. Sunset, USA, 234 p
Calderon AA, Garciaflorenciano E, Munoz R, Barcelo AR (1992) Gamay grapevine peroxidase its role in vacuolar anthocyani(di)n degradation. VITIS 31(3):139–147
Cheynier V, Souquet JM, Kontek A, Moutounet M (1994) Anthocyanin degradation in oxidizing grape musts. J Sci Food Agric 66:283–288
Davies KM, Schwinn KE, Deroles SC, Manson DG, Lewis DH, Bloor SJ, Bradley JM (2003) Enhancing anthocyanin production by altering competition for substrate between flavonol synthase and dihydroflavonol 4-reductase. Euphytica 131:259–268
Dela G, Or E, Ovadia R, Nissim-Levi A, Weiss D, Oren-Shamir M (2003) Changes in anthocyanin concentration and composition in ’Jaguar’ rose flowers due to transient high-temperature conditions. Plant Sci 164:333–340
Dooner HK, Robbins TP, Jorgensen RA (1991) Genetic and developmental control of anthocyanin biosynthesis. Annu Rev Genet 25:173–199
van Doorn WG, Harkema H, Song JS (1995) Water relations and senescence of cut Iris flowers: effects of cycloheximide. Postharvest Biol Technol 5:345–351
Dougall DK, Vogelien DL (1987) The stability of accumulated anthocyanin in suspension-cultures of the parental line and high and low accumulating subclones of wild carrot. Plant Cell Tiss Org 8(2):113–123
Halevy AH (1985) Handbook of Flowering. CRC, Boca Raton, pp 85–88
Heide OM (1963) Effect of temperature and day-length on flower initiation of Brunfelsia calycina (Hook) benth. Physiol Plant 16:104–109
Holton TA, Cornish EC (1995) Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 7:1071–1083
Jones RB, Serek M, Kuo CL, Reid MS (1994) The effect of protein synthesis inhibition on petal senescence in cut bulb flowers. J Am Soc Hortic Sci 119:1243–1247
Jonsson LMV, Donker-Koopman WE, Schram AW (1984) Turnover of anthocyanins and tissue compartmentation of anthocyanin biosynthesis in flowers of Petunia hybrida. J Plant Physiol 115:29–37
Kader F, Rovel B, Girardin M, Metche M (1997) Mechanism of browning in fresh highbush blueberry fruit (Vaccinium corymbosum L) Role of blueberry polyphenol oxidase, chlorogenic acid and anthocyanins. J Sci Food Agric 74(1):31–34
Kader F, Haluk JP, Nicolas JP, Metche M (1998) Degradation of cyanidin 3-glucoside by blueberry polyphenol oxidase: kinetic studies and mechanisms. J Agric Food Chem 46:3060–3065
Kader F, Nicolas JP, Metche M (1999) Degradation of pelargonidin 3-glucoside in the presence of chlorogenic acid and blueberry polyphenol oxidase. J Sci Food Agric 79:517–522
Mazza G, Miniati E (1993) Color stabilization and intensification. In: Mazza G, Miniati E (eds) Anthocyanins in Fruits, Vegetables, and Grains. CRC Press, Boca Raton, pp 1–20
Mol J, Grotewold E, Koes R (1998) How genes paint flowers and seeds. Trends Plant Sci 3:212–217
Nissim-Levi A, Kagan S, Ovadia R, Oren-Shamir M (2003) Effects of temperature, UV-light and magnesium on anthocyanin pigmentation in cocoplum leaves. J Hortic Sci Biotechnol 78:61–64
Oren-Shamir M, Levi-Nissim A (1997a) Temperature effects on the leaf pigmentation of Cotinus coggygria ‘Royal Purple’. J Hortic Sci 72:425–432
Oren-Shamir M, Levi-Nissim A (1997b) UV-light effect on the leaf pigmentation of Cotinus coggygria ‘Royal Purple’. Sci Hortic 71:59–66
Oren-Shamir M, Nissim-Levi A (1999) Temperature and gibberellin effects on growth and anthocyanin pigmentation in Photinia leaves. J Hortic Sci Biotechnol 74:355–360
Oren-Shamir M, Dela G, Ovadia R, Nissim-Levi A, Philosoph Hadas S, Meir S (2001) Differentiation between petal blueing and senescence of cut ‘Mercedes’ rose flowers. J Hortic Sci Biotechnol 76:195–200
Panavas T, Rubinstein B (1998) Oxidative events during programmed cell death of daylily (Hemerocallis hybrid) petals. Plant Sci 133:125–138
Quattrocchio F, Wing JF, van der Woude K, Mol JNM, Koes R (1998) Analysis of bHLH and MYB domain proteins: species-specific regulatory differences are caused by divergent evolution of target anthocyanin genes. Plant J 13:475–488
Raynal J, Moutounet M, Souquet JM (1989) Intervention of phenolic compounds in plum technology 1 changes during drying. J Agric Food Chem 37:1046–1050
Sarni P, Fulcrand H, Souillol V, Souquet JM, Cheynier V (1995) Mechanisms of anthocyanin degradation in grape must-like model solutions. J Sci Food Agr 69(3):385–391
Shaked-Sachray L, Weiss D, Moshe R, Nissim-Levi A, Oren-Shamir M (2002) Increased anthocyanin accumulation in aster flowers at elevated temperatures due to magnesium treatment. Physiol Plant 114:559–565
Steffens J, Harel E, Hunt MD (1994) Polyphenol oxidase In: Ellis BE, Kuroki GW, Stafford HA (eds) Genetic Engineering of Plant Secondary Metabolism. Plenum, New York and London, pp 275–305
Stephenson P, Rubinstein B (1998) Characterization of proteolytic activity during senescence in daylilies. Physiol Plant 104:463–473
Sultan SM, Farooq S (1998) Flower senescence in Iris kashmiriana Baker. Adv Hortic Sci 12:186–189
van Tunen AJ, Mol JNM (1991) Control of flavonoids synthesis and manipulation of flower color. Plant Biotechnol 2:94–125
Weiss MR (1995) Floral color change – a widespread functional convergence. Am J Bot 82:167–185
Weiss D, Halevy AH (1989) Stamens and gibberellin in the regulation of corolla pigmentation and growth in Petunia hybrida. Planta 179:89–96
Welinder KG, Justesen AF, Kjaersgard IVH, Jensen RB, Rasmussen SK, Jespersen HM, Duroux L (2002) Structural diversity and transcription of class III peroxidases from Arabidopsis thaliana. Eur J Biochem 269:6063–6081
Winkel-Shirley B (2001) Flavonoid biosynthesis: A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126:485–493
Zenner K, Bopp M (1987) Anthocyanin turnover in Sinapis-Alba L. J Plant Physiol 126:475–482
Acknowledgements
We thank Galil Nurseries for supplying the plants for this study. We also thank Prof. Joseph Kanner for many helpful discussions. This research was supported by the Chief Scientist of the Israeli Agriculture Ministry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaknin, H., Bar-Akiva, A., Ovadia, R. et al. Active anthocyanin degradation in Brunfelsia calycina (yesterday–today–tomorrow) flowers. Planta 222, 19–26 (2005). https://doi.org/10.1007/s00425-005-1509-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-005-1509-5