Abstract
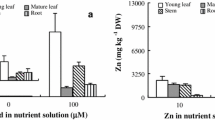
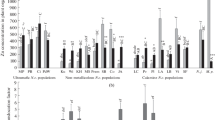
Sedum alfredii Hance can accumulate Zn in shoots over 2%. Leaf and stem Zn concentrations of the hyperaccumulating ecotype (HE) were 24- and 28-fold higher, respectively, than those of the nonhyperaccumulating ecotype (NHE), whereas 1.4-fold more Zn was accumulated in the roots of the NHE. Approximately 2.7-fold more Zn was stored in the root vacuoles of the NHE, and thus became unavailable for loading into the xylem and subsequent translocation to shoot. Long-term efflux of absorbed 65Zn indicated that 65Zn activity was 6.8-fold higher in shoots but 3.7-fold lower in roots of the HE. At lower Zn levels (10 and 100 μM), there were no significant differences in 65Zn uptake by leaf sections and intact leaf protoplasts between the two ecotypes except that 1.5-fold more 65Zn was accumulated in leaf sections of the HE than in those of the NHE after exposure to 100 μM for 48 h. At 1,000 μM Zn, however, approximately 2.1-fold more Zn was taken up by the HE leaf sections and 1.5-fold more 65Zn taken up by the HE protoplasts as compared to the NHE at exposure times >16 h and >10 min, respectively. Treatments with carbonyl cyanide m-chlorophenylhydrazone (CCCP) or ruptured protoplasts strongly inhibited 65Zn uptake into leaf protoplasts for both ecotypes. Citric acid and Val concentrations in leaves and stems significantly increased for the HE, but decreased or had minimal changes for the NHE in response to raised Zn levels. These results indicate that altered Zn transport across tonoplast in the root and stimulated Zn uptake in the leaf cells are the major mechanisms involved in the strong Zn hyperaccumulation observed in S. alfredii H.




Similar content being viewed by others
Abbreviations
- CCCP:
-
Carbonyl cyanide m-chlorophenylhydrazone
- FAD:
-
Fluorescein diacetate
- PBS:
-
Phosphate buffer solution
- HE:
-
Hyperaccumulating ecotype
- NHE:
-
Nonhyperaccumulating ecotype
References
Assunçao AGL, Schat H, Aarts MGM (2003a) Thlaspi caerulescens, an attractive model species to study heavy metal hyperaccumulation in plants. New Phytol 159:351–360
Assunçao AGL, Ten Bookun WM, Nelissen HJM, Vooijs R, Schat H, Ernst WHO (2003b) A cosegation analysis of zinc accumulation and Zn tolerance in the Zn hyperaccumulator Thlaspi caerulescense. New Phytol 159:383–390
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic elements. Biorecovery 1:81–97
Baker AJM, McGrath SP, Reeves RD, Smith JAC (2000) Metal hyperaccumulator plants: a review of the ecology and physiology resource for phytoremediation of metal-polluted soils. In: Terry N, Bañuelos G (eds) Phytoremediation of contaminated soil and water. Lewis, London, pp 85–107
Baker AJM, Walker PL (1990) Ecophysiology of metal uptake by tolerant plants. In: Shaw AJ (ed) Heavy metal tolerance in plants: evolutionary aspects. CRC, Boca Raton, pp 155–177
Bert V, Macnair MR, De Laguerie P, Saumitou-Laprade P, Petit D (2000) Zinc tolerance and accumulation in metallicolous populations of Arabidopsis halleri (Brassicaceae). New Phytol 146:225–233
Brooks RR (1998) Plants that hyperaccumulate heavy metals. CAB International, Wallingford
Brown SL, Chaney RL, Angle JS, Baker AJM (1994) Phytoremediation potential of Thlaspi caerulescens and bladder campion for zinc- and cadmium-contaminated soil. J Environ Qual 23:1151–1157
Brown SL, Chaney RL, Angle JS, Baker AJM (1995a) Zinc and cadmium uptake by hyperaccumulator Thlaspi caerulescens and metal tolerant Silene vulgaris grown on sludge-amended soils. Environ Sci Technol 29:1581–1585
Brown SL, Chaney RL, Angle JS, Baker AJM (1995b) Zinc and cadmium uptake by hyperaccumulator Thlaspi caerulescens grown in nutrient solution. J Am Soil Sci Soc 59:125–133
Cosio C, Martinoia E, Keller C (2004) Hyperaccumulation of cadmium and zinc in Thlaspi caerulescens and Arabidopsis halleri at the leaf cellular level. Plant Physiol 134:716–725
DiTomaso JM, Hart JJ, Linscott DL, Kochian LV (1992) Effect of inorganic cations and metabolic inhibitors on putrescine transport in roots of intact maize seedlings. Plant Physiol 99:508–514
Frey B, Keller C, Zierold K, Schulin R (2000) Distribution of Zn in functionally different leaf epidermal cells of the hyperaccumulator Thlaspi caerulescens. Plant Cell Environ 23:675–687
Kochian LV, Lucas WJ (1982) Potassium transport in corn roots. I. Resolution of kinetics into a saturable and linear component. Plant Physiol 70:1723–1731
Krämer U, Cotter-Howels JD, Charnock JM, Baker AJM, Smith JAC (1996) Free histidine as a metal chelator in plants that accumulate nickel. Nature 379:635–638
Küpper H, Zhao FJ, McGrath SP (1999) Cellular compartmentation of zinc in leaves of the hyperaccumulator Thlaspi caerulescens. Plant Physiol 119:305–311
Küpper H, Lombi E, Zhao FJ, McGrath SP (2000) Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta 212:75–84
Lasat MM, Baker AJM, Kochian M (1996) Physiological characterization of root Zn2+ absorption and translocation to shoots in hyperaccumulator and non-hyperaccumulator species of Thlaspi. Plant Physiol 112:1715–1722
Lasat MM, Baker AJM, Kochian LV (1998) Altered Zn compartmentation in the root symplasm and stimulated Zn absorption into the leaf as mechanisms involved in Zn hyperaccumulation in Thlaspi caerulescens. Plant Physiol 118:875–883
Li TQ, Yang XE, He ZL, Yang JY (2005a) Root morphology and Zn2+ uptake kinetics of the Zn hyperaccumulator of Sedum alfredii Hance. J Int Plant Biol 47:927–934
Li TQ, Yang XE, Jin XF, He ZL, Stoffella PJ (2005b) Root responses and metal accumulation in two contrasting ecotypes of Sedum alfredii Hance under lead and zinc toxic stress. J Environ Sci Health (Part A) 40:1081–1096
Lee KC, Adeline K, Loh CS, Wong SM (2001) Cucurbit protoplast isolation for the study of plant virus replication. J Virol Methods 91:21–27
Ma JF, Hiradate S, Nomoto K, Iwashita (1997a) Internal detoxification mechanism of Al in Hydrangea. Identification of Al form in the leaves. Plant Physiol 117:753–759
Ma JF, Zheng SJ, Hiradate S, Matsumoto H (1997b) Detoxifying aluminum with buckwheat. Nature 390:569–570
Ma JF, Ryan PR, Delhaize E (2001) Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci 6:273–278
Macklon AES, Ron MM, Sim A (1990) Cortical cell fluxes of ammonium and nitrate in excised root segments of Allium cepa L: studies using 15N. J Exp Bot 41:359–370
Macnair MR, Baker AJM (1994) Metal tolerance in plants: evolutionary aspects. In: Farago ME (ed) Plants and the chemical elements. VCH, Weinheim, pp 68–86
McGrath SP, Zhao FJ (2003) Phytoextraction of metals and metalloids from contaminated soils. Curr Opin Biotechnol 14:277–282
Nriagu JO, Pacyna JM (1988) Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 333:134–139
Pence NS, Larsen PB, Ebbs SD, Letham DLD, Lasat MM, Garvin DF, Eide D, Kochian LV (2000) The molecular physiology of heavy metal transport in the Zn/Cd hyperaccumulator Thlaspi caerulescens. Proc Natl Acad Sci USA 97:4956–4960
Pierce WS, Higinbotham N (1970) Compartments and fluxes of K+, Na+, and Cl− in Avena coleoptile cells. Plant Physiol 46:666–673
Piñeros MA, Kochian LV (2003) Differences in whole-cell and single-channel ion currents across the plasma membrane of mesophyll cells from two closely related Thlaspi species. Plant Physiol 131:583–594
Rauser WE (1987) Compartmental efflux analysis and removal of extracellular cadmium from roots. Plant Physiol 85:62–65
Rauser WE (1990) Phytochelatins. Ann Rev Biochem 59:61–86
Salt DE, Prince RC, Baker AJM, Raskin I, Pickering IJ (1999) Zinc ligands in the metal hyperaccumulator Thlaspi caerulescens as determined using X-ray absorption spectroscopy. Environ Sci Technol 33:713–717
Santa Maria GE, Cogliatti DH (1988) Bidirectional Zn-fluxes and compartmentation in wheat seedling roots. J Plant Physiol 132:312–315
Thornton B (1991) Indirect compartmental analysis of copper in live ryegrass roots: comparison with model systems. J Exp Bot 42:183–188
Van der Lelie N, Schwitzguebel JP, Glass DJ, Vangronsveld J, Baker AJM (2001) Assessing phytoremediation’s progress in the United States and Europe. Environ Sci Technol 35:446A-452A
Vázquez MD, Poschenrieder C, Barceló J, Baker AJM, Hatton P, Cope GH (1994) Compartmentation of zinc in roots and leaves of the zinc hyperaccumulator Thlaspi caerulescens J & C Presl. Bot Acta 107:243–250
Verkleij JAC, Schat H (1989) Mechanisms of metal tolerance in higher plants. In: Shaw AJ (ed) Heavy metal tolerance in plants: evolutionary aspects. CRC, Boca Raton, pp 179–193
White CW, Baker FD, Chaney RL, Decker AM (1981) Metal complexation in xylem fluid II. Theoretical equilibrium model and computational computer program. Plant Physiol 67:301–310
Yang XE, Long XX, Ni WZ (2001) Zinc tolerance and hyperaccumulation in a new ecotype of Sedum alfredii H. Acta Phytoecol Sin 25:670–677
Yang XE, Long XX, Ni WZ (2002) Sedum alfredii H—a new ecotype of Zn-hyperaccumulator plant species native to China. Chin Sci Bull 47:1003–1006
Yang XE, Long XX, Ye HB, He ZL, Calvert DV, Stoffella PJ (2004) Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii H). Plant Soil 259:181–189
Yang XE, Feng Y, He ZL, Stoffella JP (2005) Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation. J Trace Elem Med Biol 18:339–353
Ye HB, Yang XE, He B, Long XX (2003) Growth response and metal accumulation of Sedum alfredii to Cd/Zn complex-polluted ion levels. Acta Bot Sin 45:1030–1036
Zhao FJ, Lombi E, Breedon T, McGrath SP (2000) Zinc hyperaccumulation and cellular distribution in Arabidopsis halleri. Plant Cell Environ 23:507–514
Acknowledgements
This study was in part supported by the National Natural Science Foundation of China (#20277035), the Natural Science Foundation of Zhejiang Province (#Z504219), and the Program for Changjiang Scholar by Education Ministry of China. The authors are grateful to Prof. Alan Baker for his critical comments and English correction of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, X., Li, T., Yang, J. et al. Zinc compartmentation in root, transport into xylem, and absorption into leaf cells in the hyperaccumulating species of Sedum alfredii Hance. Planta 224, 185–195 (2006). https://doi.org/10.1007/s00425-005-0194-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-005-0194-8