Abstract
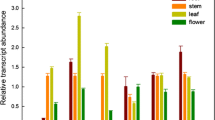
AtOPT promoter-GUS fusions were constructed for six of the nine known, putative oligopeptide transporters (OPTs) in Arabidopsis thaliana and used to examine AtOPT expression at various stages of plant development. AtOPT1, AtOPT3, AtOPT4, AtOPT6 and AtOPT7 were expressed in the embryonic cotyledons prior to root radicle emergence. Except for AtOPT8, which gave weak expression, all AtOPTs were strongly expressed in post-germinative seedlings with strongest expression in vascular tissues of cotyledons and hypocotyls. Preferential expression of AtOPTs in vascular tissues was also observed in cotyledons, leaves, hypocotyls, roots, flowers, siliques, and seed funiculi of seedlings and adult plants. Differential tissue-specific expression was observed for specific AtOPTs. For example, AtOPT1, AtOPT3 and AtOPT8 were uniquely expressed in pollen. Only AtOPT1 was expressed in growing pollen tubes, while only AtOPT6 was observed in ovules. AtOPT8 was transiently expressed in seeds during early stages of embryogenesis. Iron limitation was found to enhance expression of AtOPT3. These data suggest distinct cellular roles for specific AtOPTs including nitrogen mobilization during germination and senescence, pollen tube growth, pollen and ovule development, seed formation and metal transport.






Similar content being viewed by others
References
Bechtold N, Pelletier G (1998) In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol Biol 82:259–266
Becker DM, Fikes JD, Guarente L (1991) A cDNA encoding a human CCAAT- binding protein cloned by functional complementation in yeast. Proc Natl Acad Sci USA 88:1968–1972
Bewley JD (1997) Seed germination and dormancy. Plant Cell 9:1055–1066
Blackmore CG, McNaughton PA, van Heen HW (2001) Multidrug transporters in prokaryotic and eucaryotic cells: physiological functions and transport mechanisms. Mol Membr Biol 18:97–103
Bogs J, Bourbouloux A, Cagnac O, Wachter A, Rausch T, Delrot S (2003) Functional characterizationa and expression analysis of a glutathione transporter, BjGT1, from Brassica juncea: evidence for heavy metal regulation by heavy metal exposure. Plant Cell Environ 26:1703–1711
Bourbouloux A, Shahi P, Chakladar A, Delrot S, Bachhawat AK (2000) Hgt1p, a high affinity glutathione transporter from the yeast Saccharomyces cerevisiae. J Biol Chem 275:13259–13265
Brown RC, Lemmon BE, Nguyen H, Olsen O-A (1999) Development of endosperm in Arabidopsis thaliana. Sex Plant Reprod 12:32–42
Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, Pink D (2003) The molecular analysis of leaf senescence-a genomics approach. Plant Biotech J 1:3–22
Cagnac O, Bourbouloux A, Chakrabarty D, Zhang M-Y, Delrot S (2004) AtOPT6 transports glutathione derivatives and is induced by primisulfuron. Plant Physiol 135:1378–1387
Chiang C-S, Stacey G, Tsay Y-F (2004) Mechanisms and functional properties of two peptide transporters, AtPTR2 and fPTR2. J Biol Chem 279:30150–30157
Cho K, Zusman DR (1999) Sporulation timing in Myxococcus xanthus is controlled by the espAB locus. Mol Microbiol 34:714–725
Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat J-F, Walker EL (2001) Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 409:346–349
Detmers FJM, Lanfermiejer FV, Poolman B (2001) Peptides and ATP-binding cassette peptide transporters. Res Microbiol 152: 245–258
DiDonato RJ Jr, Roberts LA, Sanderson T, Eisley RB, Walker EL (2004) Arabidopsis Yellow Stripe-Like2 (YSL2): a metal regulated gene encoding a plasma membrane transporter of nicotianamine-metal complexes. Plant J 39:403–414
Dietrich D, Hammes U, Thor K, Suter-Grotemeyer M, Flückiger R, Slusarenko AJ, Ward JM, Rentsch D (2004) AtPTR1, a plasma membrane peptide transporter expressed during seed germination and in vascular tissue of Arabidopsis. Plant J 40:488–499
Frommer WB, Hummel S, Rentsch D (1994) Cloning of an Arabidopsis histidine transporting protein related to nitrate and peptide transporters. FEBS Lett 347:185–189
Hajdukiewicz P, Svab Z, Maliga P (1994) The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol 25:989–994
Hauser M, Donhardt AM, Barnes D, Naider F, Becker JM (2000) Enkephalines are transported by a novel eukaryotic peptide uptake system. J Biol Chem 275:3037–3041
Higgins CF, Payne JW (1980) Transport and utilization of amino acids and peptides by higher plants. In: Payne JW (ed) Microorganisms and nitrogen sources. Wiley, New York, pp 609–637
Higgins CF, Payne JW (1981) The peptide pools of germinating barley grains: relation to hydrolysis and transport of storage proteins. Plant Physiol 67:785–792
Higgins CF, Payne JW (1982) Plant Peptides. In: Boulder AD, Parthier B (eds) Encyclopedia of plant physiology, vol 14. Springer, Berlin Heidelberg New York, pp 438–458
Hirner B, Fischer WN, Rentsch D, Kwart M, Frommer WB (1998) Developmental control of H+/amino acid permease gene expression during seed development of Arabidopsis. Plant J 14:535–544
Hörtensteiner S, Feller U (2002) Nitrogen metabolism and remobilization during senescence. J Exp Bot 53:927–937
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucoronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Koh S, Donhardt AM, Sharp J, Naider F, Becker JM, Stacey G (2002) An oligopeptide transporter gene family in Arabidopsis thaliana. Plant Physiol 128:21–29
Koike S, Inoue H, Mizuno D, Takahashi H, Mori S, Nishizawa NK (2004) OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the pollen. Plant J 39:415–424
Liman ER, Tytgat J, Hess P (1992) Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9:861–871
Lubkowitz MA, Hauser L, Breslav M, Naider F, Becker JM (1997) An oligopeptide transport gene from Candida albicans. Microbiol 143:387–396
Lubkowitz MA, Barnes D, Breslav M, Burchfield A, Naider F, Becker JM (1998) Schizosaccharomyces pombe isp4 encodes a transporter representing a novel family of oligopeptide transporters. Mol Microbiol 28:729–741
Mae T, Makino A, Ohira K (1983) Changes in the amounts of ribulose biphosphate carboxylase synthesized and degraded during the life span of rice leaf. Plant Cell Physiol 24:1079–1086
Masrchner H, Römheld V, Ossenberg-Neuhaus H (1982) Rapid method for measuring changes in pH and reducing processes along roots of intact plants. Z Pflanzenphysiol 105:407–416
Miranda M, Borisjuk L, Tewes A, Dietrich D, Rentsch D, Weber H, Wobus U (2003) Peptide and amino acid transporters are differentially regulated during seed development and germination in Faba Bean. Plant Physiol 132:1950–1960
Müntz K, Belozersky MA, Dunaevsky YE, Schlereth A, Tiedemann J (2001) Stored proteinases and the initiation of storage protein mobilization in seeds during germination and seedling growth. J Exp Bot 52:1741–1752
Olsen O-A (2004) Nuclear endosperm development in cereals and Arabidopsis thaliana. Plant Cell 16:S214–S227
Rea PA, Li ZS, Lu YP, Drozdowicz YM, Martinoia E (1998) From vacuolar GS-X pumps to multispecific ABC transporters. Ann Rev Plant Physiol Plant Mol Biol 49:727–760
Roberts LA, Pierson AJ, Panaviene Z, Walker EL (2004) Yellow Stripe1. Expanded roles for the Maize iron-phytosiderophore transporter. Plant Physiol 135:112–120
Rubio F, Gassmann W, Schroeder JI (1995) Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 270:1660–1663
Sanders PM, Bui AQ, Wetterings K, McIntire KN, Hsu Y-C, Lee PY, Troung MT, Beals TP, Goldberg RB (1999) Anther developmental defects in Arabidopsis thaliana male sterile mutants. Sex Plant Reprod 11:297–322
Schaaf G, Ludewig U, Erenoglu BE, Mori S, Kitahara T, Wiren NV (2004) ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. J Biol Chem 279:9091–9096
Schlereth A, Standhardt D, Mock HP, Müntz K (2001) Stored cysteine proteinases start globulin breakdown in protein bodies of embryonic axes and cotyledons of germinating vetch (Vicia sativa L.) seeds. Planta 212:718–727
Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Schölkopf B, Weigel D, Lohmann J (2005) A gene expression map of Arabidopsis thaliana development. Nat Genet 37(5):501–506
Smyth DR, Bowman JL, Meyerowitz (1990) Early flower development in Arabidopsis. Plant Cell 2:755–767
Song W, Steiner HY, Zang L, Naider F, Becker JM, Stacey G (1996) Cloning of a second Arabidopsis peptide transport gene. Plant Physiol 110:171–178
Stacey G, Koh S, Granger C, Becker JM (2002a) Peptide transport in plants. Trends Plant Sci 7:257–263
Stacey M, Koh S, Becker J, Stacey G (2002b) AtOPT3, a member of the oligopeptide transporter family, is essential for embryo development in Arabidopsis. Plant Cell 14:2799–2811
Steiner HY, Song W, Zhang L, Naider F, Becker JM, Stacey G (1994) An Arabidopsis peptide transporter is a member of a new class of membrane transport proteins. Plant Cell 6:1289–1299
Stessman D, Miller A, Spalding M, Rodermel S (2002) Regulation of photosynthesis during Arabidopsis leaf development in continuous light. Photosynth Res 72:27–37
Tiedemann J, Neubohn B, Müntz K (2000) Different functions of vicilin and legumin are reflected in the histopattern of globulin mobilization during germination of vetch (Vicia sativa L.). Planta 211:1–12
Tsay Y-F, Schroeder JI, Feldman KA, Crawford NM (1993) The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 72:705–713
Walker-Smith DJ, Payne JW (1984) Characteristics of the active transport of peptides and amino acids by germinating barley embryos. Planta 162:159–165
Walker-Smith DJ, Payne JW (1985) Synthesis of the peptide transport carrier of the barley scutellum during early stages of germination. Planta 164:550–556
Waterworth WM, West CE, Bray CM (2000) The barley scutellar peptide transporter: biochemical characterization and localization to the plasma membrane. J Exp Bot 51:1201–1209
Waterworth WM, West CE, Bray CM (2001) The physiology and molecular biology of peptide transport in seeds. Seed Sci Res 11:275–284
West MAL, Harada JJ (1993) Embryogenesis in higher plants: an overview. Plant Cell 5:1361–1369
West CE, Waterworth WM, Stephens SM, Smith CP, Bray CM (1998) Cloning and functional characterization of a peptide transporter expressed in the scutellum of barley grain during the early stages of germination. Plant J 15:221–230
Williams LE, Miller AJ (2001) Transporters responsible for the uptake and partitioning of of nitrogenous solutes. Annu Rev Plant Mol Biol 52:659–688
Wintz H, Fox T, Wu Y-Y, Feng V, Chen W, Chang H-S, Zhu T, Vulpe C (2003) Expression profiles of Arabidopsis thaliana in mineral deficiencies reveal novel transporters involved in metal homeostasis. J Biol Chem 278:47644–47653
von Wiren N, Mori S, Masrchner H, Romheld V (1994) Iron inefficiency in maize mutant ys1 (Zea mays L. cv Yellow-Stripe) is caused by a defect in uptake of iron phytosiderophores. Plant Physiol 106:71–77
Yen M-R, Tseng Y-H, Saier MH Jr (2001) Maize Yellow Stripe1, an iron phytosiderophore uptake transporter, is a member of the oligopeptide transporter (OPT) family. Microbiol 147:2881–2883
Zhang MY, Bourbouloux A, Cagnac O, Shrikanth CV, Rentsch D, Bachhawat AK, Delrot S (2004) A novel family of transporters mediating the transport of glutathione derivatives in plants. Plant Physiol 134:482–491
Zhou J, Theodoulou FL, Ingemarsson B, Miller AJ (1998) Cloning and functional characterization of a Brassica napus transporter that is able to transport nitrate and histidine. J Biol Chem 273:12017–12023
Acknowledgements
We thank Dr. Elizabeth E. Rogers and Dr. Bruce A McClure for their valuable technical suggestions and helpful discussions. We also thank Dr. Tom Guilfoyle for giving us access to his spectrofluorometer. This work was supported in part by the University of Missouri-Columbia Life Sciences Post-Doctoral Fellowship (H.O.) and by the National Science Foundation (grant MCB-0235286 to G.S. and W.G.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stacey, M.G., Osawa, H., Patel, A. et al. Expression analyses of Arabidopsis oligopeptide transporters during seed germination, vegetative growth and reproduction. Planta 223, 291–305 (2006). https://doi.org/10.1007/s00425-005-0087-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-005-0087-x