Abstract
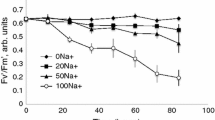
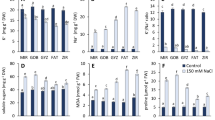
Considering the physiological significance of Mg homeostasis in plants, surprisingly little is known about the molecular and ionic mechanisms mediating Mg transport across the plasma membrane and the impact of Mg availability on transport processes at the plasmalemma. In this study, a non-invasive ion-selective microelectrode technique (MIFE) was used to characterize the effects of Mg availability on the activity of plasma membrane H+, K+, Ca2+, and Mg2+ transporters in the mesophyll cells of broad bean (Vicia faba L.) plants. Based on the stoichiometry of ion-flux changes and results of pharmacological experiments, we suggest that at least two mechanisms are involved in Mg2+ uptake across the plasma membrane of bean mesophyll cells. One of them is a non-selective cation channel, also permeable to K+ and Ca2+. The other mechanism, operating at concentrations below 30 μM, was speculated to be an H+/Mg+ exchanger. Experiments performed on leaves grown at different levels of Mg availability (from deficient to excessive) showed that Mg availability has a significant impact on the activity of plasma-membrane transporters for Ca2+, K+, and H+. We discuss the physiological significance of Mg-induced changes in leaf electrophysiological responses to light and the ionic mechanisms underlying this process.









Similar content being viewed by others

Abbreviations
- ROS:
-
Reactive oxygen species
- NSCC:
-
Non-selective cation channel
References
Allen GJ, Sanders D (1997) Vacuolar ion channels of higher plants. Adv Bot Res 25:218–252
Amalou Z, Gibrat R, Trouslot P, d’Auzac J (1994) Solubilization and reconstitution of the Mg2+/2H+ antiporter of the lutoid tonoplast from Hevea brasiliensis latex. Plant Physiol 106:79–85
Babourina O, Newman I, Shabala S (2002) Blue light-induced kinetics of H+ and Ca2+ fluxes in etiolated wild-type and phototropin-mutant Arabidopsis seedlings. Proc Natl Acad Sci U S A 99:2433–2438
Bruggemann LI, Pottosin II, Schonknecht G (1999) Cytoplasmic magnesium regulates the fast activating cation channel. J Exp Bot 50:1547–1552
Cakmak I (1994) Activity of ascorbate-dependent H2O2-scavenging enzymes and leaf chlorosis are enhanced in magnesium- and potassium-deficient leaves, but not in phosphorus-deficient leaves. J Exp Bot 278:1259–1266
Cakmak I, Hengeler C, Marschner H (1994) Changes in phloem export of sucrose in leaves in response to phosphorus, potassium and magnesium deficiency in bean plants. J Exp Bot 278:1251–1257
Dietz KJ, Schramm M, Lang B, Lanzl-Schramm A, Durr C, Martinoia E (1992) Characterization of the epidermis from barley primary leaves. II. The role of the epidermis in ion compartmentation. Planta 187:431–437
Elzenga JTM (1997) Kinetic properties of blue light pulse-induced acidification by leaf epidermal cells of pea. Plant Physiol 114:1474–1474
Elzenga JTM, Prins HBA, Van Volkenburgh E (1995) Light-induced membrane potential changes of epidermal and mesophyll cells in growing leaves of Pisum sativum. Planta 197:127–134
Elzenga JTM, Staal M, Prins HBA (1997) Calcium-calmodulin signalling is involved in light-induced acidification by epidermal leaf cells of pea, Pisum sativum L. J Exp Bot 48:2055–2061
Fischer ES (1997) Photosynthetic irradiance curves of Phaseolus vulgaris under moderate or severe magnesium deficiency. Photosynthetica 33:385–390
Fraichard A, Trossat C, Perotti E, Pugin A (1996) Allosteric regulation by Mg2+ of the vacuolar H+-PPase from Acer pseudoplatanus cells. Ca2+/Mg2+ interactions. Biochimie 78:259–266
Gardner RC (2003) Genes for magnesium transport. Curr Opin Plant Biol 6:263–267
Garnett TP, Shabala SN, Smethurst PJ, Newman IA (2003) Kinetics of ammonium and nitrate uptake by eucalypt roots and associated proton fluxes measured using ion selective microelectrodes. Funct Plant Biol 30:1165–1176
Hansen U-P, Moldaenke C, Tabrizi H, Ramm D (1993) The effect of transthylakoid proton uptake on cytosolic pH and the imbalance of ATP and NADPH/H+ production as measured by CO2- and light-induced depolarisation of the plasmalemma. Plant Cell Physiol 34:681–695
Hariadi Y, Shabala S (2004) Screening broad beans (Vicia faba L.) for magnesium deficiency. 1. Growth characteristics, visual deficiency symptoms and plant nutritional status. Funct Plant Biol 31:529–537
Haynes WJ, Kung C, Saimi Y, Preston RR (2002) An exchanger-like protein underlies the large Mg2+ current in Paramecium. Proc Natl Acad Sci U S A 99:15717–15722
Hinnah SC, Wagner R (1998) Thylakoid membranes contain a high-conductance channel. Eur J Biochem 253:606–613
Horlitz M, Klaff P (2000) Gene-specific trans-regulatory functions of magnesium for chloroplast mRNA stability in higher plants. J Biol Chem 275: 35638–35645
Ishijima S, Uchlbori A, Takagi H, Maki R, Ohnishi M (2003) Light-induced increase in free Mg2+ concentration in spinach chloroplasts: measurement of free Mg2+ by using a fluorescent probe and necessity of stromal alkalinization. Arch Biochem Biophys 412:126–132
Lavon R, Goldschmidt EE (1999) Effect of potassium, magnesium, and calcium deficiencies on nitrogen constituents and chloroplast components in Citrus leaves. J Am Soc Hortic Sci 124:158–162
Li L, Tutone AF, Drummond RS, Gardner RC, Luan S (2001) A novel family of magnesium transport genes in Arahidopsls. Plant Cell 13:2761–2775
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, San Diego
Newman IA (2001) Ion transport in roots: measurement of fluxes using ion-selective microelectrodes to characterize transporter function. Plant Cell Environ 24:1–14
Nicoll DA, Longoni S, Philipson KD (1990) Molecular cloning and functional expression of the cardiac sarcolemmal Na+–Ca2+ exchanger. Science 250:562–565
Pei ZM, Ward JM, Schroeder JI (1999) Magnesium sensitizes slow vacuolar channels to physiological cytosolic calcium and inhibits fast vacuolar channels in Fava bean guard cell vacuoles. Plant Physiol 121:977–986
Pineros M, Tester MA (1997) Calcium channels in higher plant cells: selectivity, regulation and pharmacology. J Exp Bot 48:551–577
Pottosin II, Muniz J (2002) Higher plant vacuolar ionic transport in the cellular context. Acta Bot Mex 60:37–77
Pottosin II, Schönknecht G (1996) Ion channel permeable for divalent and monovalent cations in native spinach thylakoid membranes. J Membr Biol 152:223–233
Pottosin II, Tikhonova LI, Hedrich R, Schönknecht G (1997) Slowly activating vacuolar ion channel cannot mediate Ca2+-induced Ca2+ release. Plant J 12:1387–1398
Remis D, Bulychev AA, Kurella GA (1986) The electrical and chemical components of the protonmotive force in chloroplasts as measured with capillary and pH-sensitive microelectrodes. Biochim Biophys Acta 852:68–73
Ridolfi M, Garrec J-P (2000) Consequences of an excess Al and a deficiency in Ca and Mg for stomatal functioning and net carbon assimilation of beech leaves. Ann For Sci 57:209–218
Shabala S (2000) Ionic and osmotic components of salt stress specifically modulate net ion fluxes from bean leaf mesophyll. Plant Cell Environ 23:825–838
Shabala S, Lew RR (2002) Turgor regulation in osmotically stressed Arabidopsis epidermal root cells. Direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiol 129:290–299
Shabala SN, Newman IA (1999) Light-induced transient changes in hydrogen, calcium, potassium, and chloride ion fluxes and concentrations from the mesophyll and epidermal tissues of bean leaves. Understanding the ionic basis of light-induced bioelectrogenesis. Plant Physiol 119:1115–1124
Shabala SN, Newman IA, Morris J (1997) Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol 113:111–118
Shabala S, Babourina O, Newman IA (2000) Ion-specific mechanisms of osmoregulation in bean mesophyll cells. J Exp Bot 51:1243–1253
Shabala S, Shabala L, Van Volkenburgh E (2003) Effect of calcium on root development and root ion fluxes in salinised barley seedlings. Funct Plant Biol 30:507–514
Shaul O (2002) Magnesium transport and function in plants: the tip of the iceberg. BioMetals 15:309–323
Shaul O, Hilgemann DW, Almeida-Engler J, Van Montagu M, Inze D, Galili G (1999) Cloning and characterization of a novel Mg2+/H+ exchanger. EMBO J 18:3973–3980
Spalding EP, Slayman CL, Goldsmith MHM, Gradmann D, Bertl A (1992) Ion channels in Arabidopsis plasma membrane. Transport characteristics and involvement in light-induced voltage changes. Plant Physiol 99:96–102
Sun OJ, Payn TW (1999) Magnesium nutrition and photosynthesis in Pinus radiata: clonal variation and influence of potassium. Tree Physiol 19:535–540
Tikhonova LI, Pottosin II, Dietz K-J, Schönknecht G (1997) Fast-activating cation channel in barley mesophyll vacuoles. Inhibition by calcium. Plant J 11:1059–1070
Tisdale SL, Nelson WL, Beaton JD, Havlin JL (199’3) Soil Fertility and Fertilizers. 5th edn. Prentice Hall, New Jersey
White PJ, Pineros M, Tester M, Ridout MS (2000) Cation permeability and selectivity of a root plasma membrane calcium channel. J Membr Biol 174:71–83
Yazaki Y, Asukawagawa N, Ishikawa Y, Ohta E, Sakata M (1988) Estimation of cytoplasmic free Mg2+ levels and phosphorylation potentials in mung bean root tips by in vivo 31P NMR spectroscopy. Plant Cell Physiol 29:919–924
Yin ZH, Huve K, Heber U (1996) Light-dependent proton transport into mesophyll vacuoles of leaves of C3 plants as revealed by pH-indicating fluorescent dyes—a reappraisal. Planta 199:9–17
Acknowledgements
This work was supported by an ARC Large Grant (A00001144) to Dr S. Shabala. My sincere thanks to Dr Richard Gardner and Prof. Igor Pottosin for helpful discussion and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shabala, S., Hariadi, Y. Effects of magnesium availability on the activity of plasma membrane ion transporters and light-induced responses from broad bean leaf mesophyll. Planta 221, 56–65 (2005). https://doi.org/10.1007/s00425-004-1425-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-004-1425-0