Abstract
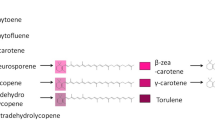
The plant carotenoid biosynthetic pathway to cyclic carotenes proceeds via carotene precursors in cis configuration. Involvement of individual isomers was elucidated by genetic complementation of desaturations and in vitro reactions of the corresponding enzyme. Determination of substrate and product specificity of phytoene and ζ-carotene desaturase revealed that 15-cis-phytoene is converted to 9,15,9′-tricis-ζ-carotene with 15,9′-dicis-phytofluene as intermediate by the first desaturase. Prior to a subsequent conversion by ζ-carotene desaturase, the 15-cis double bond of 9,15,9′-tricis-ζ-carotene has to be (photo)isomerized to all-trans. Then, the resulting 9,9′-dicis-ζ-carotene is utilized by ζ-carotene desaturase via 7,9,9′-tricis-neurosporene to 7,9,7′,9′-tetracis-lycopene. Other ζ-carotene isomers that are assumed to be spontaneous isomerization products were not converted, except for the asymmetric 9-cis-ζ-carotene. This isomer is desaturated only to 7,9-dicis-neurosporene resembling a dead-end of the pathway. Prolycopene, the product of the desaturation reactions, is finally isomerized by a specific isomerase to all-trans-lycopene, which is a prerequisite for cyclization to β-carotene. The 5-cis-lycopene and the 9-cis-and 13-cis-β-carotene isomers detected in leaves are thought to originate independently from cis precursors by non-enzymatic isomerization of their all-trans forms.





Similar content being viewed by others
Abbreviations
- CMPA :
-
2-(4-Chlorophenylthio)-dimethylamine
- Pds :
-
Phytoene desaturase
- Pfl :
-
Phytofluene
- Zds :
-
ζ-Carotene desaturase
- ζ :
-
ζ-Carotene
References
Albrecht M, Klein A, Hugueney P, Sandmann G, Kuntz M (1995) Molecular cloning and functional expression in E.coli of a novel plant enzyme mediating ζ-carotene desaturation. FEBS Lett 372:199–202
Bartley GE, Scolnik PA, Beyer P (1999) Two Arabidopsis thaliana carotene desaturases, phytoene desaturase and ζ-carotene desaturase, expressed in Escherichia coli, catalyze a poly-cis pathway to yield pro-lycopene. Eur J Biochem 259:396–403
Bejarano ER, Govind NS, Cerda-Olmedo E (1987) ζ-Carotene and other carotenoids in a Phycomyces mutant. Phytochemistry 26:2251–2254
Beyer P, Mayer M, Kleinig H (1989) Molecular oxygen and the state of geometric isomerism of intermediate are essential in the carotene desaturation and cyclization reactions in daffodil chromoplasts. Eur J Biochem 184:141–150
Breitenbach J, Kuntz M, Takaichi S, Sandmann G (1999) Catalytic properties of an expressed and purified higher plant type ζ-carotene desaturase from Capsicum annuum. Eur J Biochem 265:376–383
Breitenbach J, Vioque A, Sandmann G (2001a) Gene sll0033 from Synechocystis 6803 encodes a carotene isomerase involved in the biosynthesis of all-E lycopene. Z Naturforsch Teil C 56:915–917
Breitenbach J, Braun G, Steiger S, Sandmann G (2001b) Chromatographic performance on a C30-bounded stationary phase of monohydroxycarotenoids with variable chain length or degree of desaturation and of lycopene isomers synthesized by various carotene desaturases. J Chromatogr A 936:59–69
Breitenbach J, Zhu C, Sandmann G (2001c) Bleaching herbicide norflurazon inhibits phytoene desaturase by competition with the cofactors. J Agric Food Chem 49:5270–5272
Britton G (1995) In: UV/Visible spectroscopy. In: Britton G, Liaaen-Jensen S, Pfander H (eds) Carotenoids, vol 1B. Birkhäuser, Basel, pp 13–62
Chollet R, Sandmann G, Diethelm R, Felix H, Milzner K, Böger P(1990) ζ-Carotene accumulation and bleaching by new pyrimidine compounds. Pestic Sci 30:326–329
Clough JM, Pattenden G (1983) Stereochemical assignment of prolycopene and other poly-Z-isomeric carotenoids in fruits of the tangerine tomato Lycopersicon esculentum var. ‘Tangella’. J Chem Soc Perkin Trans 1:3011–3018
Dachtler M, GlaserT, Kohler K, Albert K (2001) Combined HPLC-MS and HPLC-NMR on-line coupling for the separation and determination of lutein and zeaxanthin stereoisomers in spinach and in retina. Anal Chem 73:667–674
Davis JB, Jackman LM, Siddons PT, Weedon BCL (1966) Carotenoids and related compounds. Part XV. The structure and synthesis of phytoene, phytofluene, ζ-carotene, and neurosporene. J Chem Soc 1966:2154–2165
Ditta G, Schmidhauser T, Yakobson E, Lu P, Liang X-W, Finlay DR, Guiney D, Helinski DR (1985) Plasmids related to the broad host range vector pRK290, useful for gene cloning and for monitoring gene expression. Plasmid 13:149–153
Englert G (1979) Prolycopene, a tetra-cis carotene with two hindered cis double bonds. J Chem Soc Chem Commun 279:545–547
Ernst S, Sandmann G (1988) Poly-cis carotene pathway in the Scenedesmus mutant C-6D. Arch Microbiol 150:590–594
Giuliano G, Giliberto L, Rosati C (2002) Carotenoid isomerase: a tale of light and isomers. Trends Plant Sci 7:427–429
Goodwin TW (1983) Developments in carotenoid biochemistry over 40 years. Biochem Soc Trans 11:473–483
Isaacson T, Ronen G, Zamir D, Hirschberg J (2002) Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of beta-carotene and xanthophylls in plants. Plant Cell 14:333–342
Jungalwala FB, Porter JW (1965) The configuration of phytoene. Arch Biochem Biophys 110:29–299
Masamoto K, Wada H, Kaneko T, Takaichi S (2001) Identification of a gene required for cis-to-trans carotene isomerization in carotenogensis of the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol 42:1398–1402
Misawa N, Truesdale MR, Sandmann G, Fraser PD, Bird C, Schuch W, Bramley PM (1994) Expression of a tomato cDNA coding for phytoene synthase in Escherichia coli, phytoene formation in vivo and in vitro, and functional analysis of the various truncated gene products. J Biochem 116:980–985
Moss GP (1979) Physico-chemical and synthetic studies on carotenoids. Pure Appl Chem 51:507–514
Park H, Kreunen SS, Cuttriss AJ, DellaPenna D, Pogson BJ (2002) Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell 14:321–332
Pesek CA, Warthesen JJ, Taoukis PS (1990) A kinetic model for equilibration of isomeric β-carotenes. J Agric Food Chem 38:41–45
Petracek FJ, Zechmeister L (1952) Stereoisomeric phytofluenes. J Am Chem Soc 74:184–186
Römer S, Hugueney P, Bouvier F, Camara B, Kuntz M (1993) Expression of the genes encoding the early carotenoid biosynthetic enzymes in Capsicum annuum. Biochem Biophys Res Commun 196:1414–1421
Sander LC, Sharpless KE, Craft NE, Wise SA (1994) Development of engineered stationary phases for the separation of carotenoid isomers. Anal Chem 66:1667–1674
Sandmann G (1994) Phytoene desaturase: genes, enzymes and phylogenetic aspects. J Plant Physiol 143:444–447
Sandmann G (2002) Molecular evolution of carotenoid biosynthesis from bacteria to plants. Physiol Plant 116:431–440
Sandmann G, Albrecht M (1990) Accumulation of colorless carotenes and derivatives during interaction of bleaching herbicides with phytoene desaturation. Z Naturforsch Teil C 45:487–491
Stickforth P, Steiger S, Hess WR, Sandmann G (2003) A novel type of lycopene epsilon-cyclase in the marine cyanobacterium Prochlorococcus marinus MED4. Arch Microbiol 179:409–415
Zechmeister L, LeRosen AL, Went FW, Pauling L (1941) Prolycopene, a naturally occurring stereoisomer of lycopene. Proc Natl Acad Sci USA 27:468–474
Zhu C, Yamamura S, Koiwa H, Nishihara M, Sandmann G (2002) cDNA cloning and expression of carotenogenic genes during flower development in Gentiana lutea. Plant Mol Biol 48:277–285
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Breitenbach, J., Sandmann, G. ζ-Carotene cis isomers as products and substrates in the plant poly-cis carotenoid biosynthetic pathway to lycopene. Planta 220, 785–793 (2005). https://doi.org/10.1007/s00425-004-1395-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-004-1395-2