Abstract
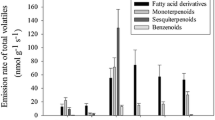
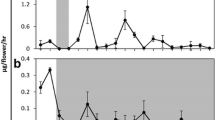
The control of rhythmic emission of floral volatiles emitted from Rosa damascena semperflorens cv. ‘Quatre Saisons’ throughout floral development under various light regimes was studied. 2-Phenylethanol was the major volatile emitted in addition to monoterpenols, oxidised monoterpenols, monoterpenes and aromatic compounds. All detected volatiles were emitted rhythmically, with maximum peaks coinciding 8–10 h into a 12-h photoperiod. For some compounds a secondary, nocturnal peak was apparent. The primary and secondary maxima both occurred at approximately 24-h intervals. Rhythms appeared to be regulated endogenously: rhythmic emission continued upon exposure to continuous light or continuous darkness, and a phase shift in emission was induced upon inversion of the photoperiod. Additionally, emission continued after flower excision. A similar profile of free volatiles was stored within the floral tissue, together with glycosidic forms of 2-phenylethanol (>99% β-d-glucoside), benzyl alcohol, citronellol and geraniol. Regression analysis indicated a significant decrease in glycosylated 2-phenylethanol through the photoperiod. These results suggest that glycosylated volatiles stored within petals may be a source of rhythmically emitted volatiles.





Similar content being viewed by others
Abbreviations
- CD :
-
Continuous darkness
- CL :
-
Continuous light
- 2-PE :
-
2-Phenylethanol
- 2-PEG :
-
2-Phenylethyl β-d-glucopyranoside
References
Ackermann IE, Banthorpe DV, Fordham WD, Kinder JP, Poots I (1989) Beta-glucosides of aroma components from petals of Rosa species—assay, occurrence, and biosynthetic implications. J Plant Physiol 134:567–572
Altenburger R, Matile P (1988) Circadian rhythmicity of fragrance emission in flowers of Hoya carnosa R. Br. Planta 174:248-252
Borgkarlson AK, Valterova I, Nilsson LA (1994) Volatile compounds from flowers of 6 species in the family Apiaceae—bouquets for different pollinators. Phytochemistry 35:111–119
Dudareva N, Murfitt LM, Mann CJ, Gorenstein N, Kolosova N, Kish CM, Bonham C, Wood K (2000) Developmental regulation in methyl benzoate biosynthesis and emission in snapdragon flowers. Plant Cell 12:949–961
Ervik F, Tollsten L, Knudsen JT (1999) Floral scent chemistry and pollination ecology in phytelephantoid palms (Arecaceae). Plant Syst Evol 217:279–297
Francis MJO, Allcock C (1969) Geranyl-β-d-glucoside: occurrence and synthesis in rose flowers. Phytochemistry 8:1339–1347
Galen C (1996) Rates of floral evolution: adaptation to bumblebee pollination in an alpine wildflower, Polemonium viscosum. Evolution 50:120–125
Hansted L, Jakobsen HB, Olsen CE (1994) Influence of temperature on the rhythmic emission of volatiles from Ribes nigrum flowers in situ. Plant Cell Environ 17:1069–1072
Helsper JPFG, Davies JA, Bouwmeester HJ, Krol AF, van Kampen MH (1998) Circadian rhythmicity in emission of volatile compounds by flowers of Rosa hybrida L. cv. Honesty. Planta 207:88–95
Helsper JPFG, Davies JA, Verstappen FWA (2002) Analysis of rhythmic emission of volatile compounds of rose flowers. In: Jackson JF, Linskens HF (eds) Molecular methods in plant analysis, vol 21. Springer, Berlin Heidelberg New York, pp 199–221
Jakobsen H, Christensen LF (2002) Diurnal changes in the concentrations of 2-phenylethyl β-d-glucopyranoside and the corresponding volatile aglycone in the tissue and headspace of Trifolium repens L. florets. Plant Cell Environ 25:773–781
Jakobsen H, Olsen C (1994) Influence of climatic factors on emission of flower volatiles in situ. Planta 192:365–371
Jones MB, Mansfield TA (1975) Circadian rhythms in plants. Sci Prog Oxford 62:103–125
Jurgens A, Webber AC, Gottsberger G (2000) Floral scent compounds of Amazonian Annonaceae species pollinated by small beetles and thrips. Phytochemistry 55:551–558
Knudsen JT, Tollsten L, Bergstrom LG (1993) Floral scents—a checklist of volatile compounds isolated by head-space techniques. Phytochemistry 33:253–280
Kolosova N, Gorenstein N, Kish CM, Dudareva N (2001) Regulation of circadian methyl benzoate emission in diurnally and nocturnally emitting plants. Plant Cell 13:2333–2347
Loughrin JH, Hamilton-Kemp TR, Andersen RA, Hildebrand DF (1991) Circadian rhythm of volatile emission from flowers of Nicotiana sylvestris and N. suaveolens. Physiol Plant 83:492–496
Loughrin JH, Hamilton-Kemp TR, Burton HR, Andersen RA, Hildebrand DF (1992) Glycosidically bound volatile components of Nicotiana sylvestris and N. suaveolens flowers. Phytochemistry 31:1537–1540
MacTavish HS, Davies NW, Menary RC (2000) Emission of volatiles from brown boronia flowers: some comparative observations. Ann Bot 86:347–354
Matile P, Altenburger R (1988) Rhythms of fragrance emission in flowers. Planta 174:242–247
Miyake T, Yamaoka R, Yahara T (1998) Floral scents of hawkmoth-pollinated flowers in Japan. J Plant Res 111:199–205
Odell E, Raguso RA, Jones KN (1999) Bumblebee foraging responses to variation in floral scent and color in snapdragons (Antirrhinum: Scrophulariaceae). Am Midl Nat 142:257–265
Oka N, Ohishi H, Hatano T, Hornberger M, Sakata K, Watanabe N (1999) Aroma evolution during flower opening in Rosa damascena Mill. Z Naturforsch Teil C 54:1–7
Omura H, Honda K, Hayashi N (2000) Floral scent of Osmanthus fragrans discourages foraging behavior of cabbage butterfly, Pieris rapae. J Chem Ecol 26:655–666
Overland L (1960) Endogenous rhythm in opening and odor of flowers of Cestrum nocturnum. Am J Bot 47:378–382
Reuveni M, Sagi Z, Evnor D, Hetzroni A (1999) β-glucosidase activity is involved in scent production in Narcissus flowers. Plant Sci 147:19–24
Schiestl F, Ayasse M, Paulus H, Erdmann D, Francke W (1997) Variation of floral scent emission and postpollination changes in individual flowers of Ophrys sphegodes subsp. sphegodes. J Chem Ecol 23:2881–2895
Watanabe N, Watanabe S, Nakajima R, Moon JH, Shimokihara K, Inagaki J, Etoh H, Asai T, Sakata K, Ina K (1993) Formation of flower fragrance compounds from their precursors by enzymatic action during flower opening. Biosci Biotechnol Biochem 57:1101–1106
Watanabe S, Hashimoto I, Hayashi K, Yagi K, Asai T, Knapp H, Straubinger M, Winterhalter P, Watanabe N (2001) Isolation and identification of 2-phenylethyl disaccharide glycosides and mono glycosides from rose flowers, and their potential role in scent formation. Biosci Biotechnol Biochem 65:442–445
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Picone, J.M., Clery, R.A., Watanabe, N. et al. Rhythmic emission of floral volatiles from Rosa damascena semperflorens cv. ‘Quatre Saisons’. Planta 219, 468–478 (2004). https://doi.org/10.1007/s00425-004-1250-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-004-1250-5