Abstract
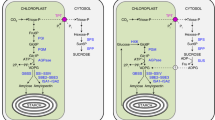
Transitory starch is formed in chloroplasts during the day and broken down at night. We investigated carbon export from chloroplasts resulting from transitory-starch breakdown. Starch-filled chloroplasts from spinach (Spinacia oleracea L. cv. Nordic IV) were isolated 1 h after the beginning of the dark period and incubated for 2.5 h, followed by centrifugation through silicone oil. Exported products were measured in the incubation medium to avoid measuring compounds retained inside the chloroplasts. Maltose and glucose made up 85% of the total exported products and were exported at rates of 626 and 309 nmol C mg−1 chlorophyll h−1, respectively. Net export of phosphorylated products was less than 5% and higher maltodextrins were not detected. Maltose levels in leaves of bean (Phaseolus vulgaris L. cv. Linden), spinach, and Arabidopsis thaliana (L.) Heynh. were low in the light and high in the dark. Maltose levels remained low and unchanged during the light/dark cycle in two starch-deficient Arabidopsis mutants, stf1, deficient in plastid phosphoglucomutase, and pgi, deficient in plastid phosphoglucoisomerase. Through the use of nonaqueous fractionation, we determined that maltose was distributed equally between the chloroplast and cytosolic fractions during darkness. In the light there was approximately 24% more maltose in the cytosol than the chloroplast. Taken together these data indicate that maltose is the major form of carbon exported from the chloroplast at night as a result of starch breakdown. We hypothesize that the hydrolytic pathway for transitory-starch degradation is the primary pathway used when starch is being converted to sucrose and that the phosphorolytic pathway provides carbon for other purposes.







Similar content being viewed by others
Abbreviations
- CAM:
-
crassulacean acid metabolism
- Chl:
-
chlorophyll
- DHAP:
-
dihydroxyacetone phosphate
- FBPase:
-
fructose bisphosphatase
- GAP:
-
glyceraldehyde-3-phosphate
- G6P:
-
glucose 6-phosphate
- PGA:
-
3-phosphoglycerate
- TPT:
-
triose phosphate translocator
- WT:
-
wild type
References
Boos W, Shuman H (1998) Maltose/maltodextrin system of Escherichia coli: transport, metabolism, and regulation. Microbiol Mol Biol Rev 62:204–229
Bouma TJ, De Visser R, Van Leeuwen PJ, DeKock MJ, Lambers H (1995) The respiratory energy requirements involved in nocturnal carbohydrate export from starch-storing mature source leaves and their contribution to dark respiration. J Exp Bot 46:1185–1194
Caspar T, Lin T-P, Monroe J, Bernhard W, Spilatro S, Preiss J, Somerville C (1989) Altered regulation of β-amylase activity in mutants of Arabidopsis with lesion in starch metabolism. Proc Natl Acad Sci USA 86:5830–5833
Caspar T, Lin T-P, Kakefuda G, Benbow L, Preiss J, Somerville C (1991) Mutants of Arabidopsis with altered regulation of starch degradation. Plant Physiol 95:1181–1188
Critchley J, Zeeman S, Takaha T, Smith AM, Smith SM (2001) A critical role for disproportionating enzyme in starch breakdown is revealed by a knockout mutation in Arabidopsis thaliana. Plant J 26:89–100
Flügge U-I (1999) Phosphate translocators in plastids. Annu Rev Plant Physiol Plant Mol Biol 50:27–45
Flügge U-I, Heldt HW (1991) Metabolite translocators of the chloroplast envelope. Annu Rev Plant Physiol Plant Mol Biol 42:129–144
Gerhardt R, Heldt HW (1984) Measurement of subcellular metabolite levels in leaves by fractionation of freeze-stopped material in nonaqueous media. Plant Physiol 75:542–547
Hattenbach A, Müller-Röber B, Nast G, Heineke D (1997) Antisense repression of both ADP-glucose pyrophosphorylase and triose phosphate translocator modifies carbohydrate partitioning in leaves. Plant Physiol 115:471–475
Häusler RE, Schlieben NH, Schulz B, Flügge U-I (1998) Compensation of decreased triose phosphate/phosphate translocator activity by accelerated starch turnover and glucose transport in transgenic tobacco. Planta 204:366–376
Häusler RE, Schlieben NH, Nicolay P, Fischer K, Fischer KL, Flügge U-I (2000a) Control of carbon partitioning and photosynthesis by the triose phosphate/phosphate translocator in transgenic tobacco plants (Nicotiana tabacum L.) I. Comparative physiological analysis of tobacco plants with antisense repression and overexpression of the triose phosphate/phosphate translocator. Planta 210:371–382
Häusler RE, Schlieben NH, Flügge U-I (2000b) Control of carbon partitioning and photosynthesis by the triose phosphate/phosphate translocator in transgenic tobacco plants (Nicotiana tabacum L.) II. Assessment of control coefficients of the triose phosphate/phosphate translocator. Planta 210:383–390
Heineke D, Kruse A, Flügge U-I, Frommer WB, Riesmeier JW, Willmitzer L, Heldt HW (1994) Effect of antisense repression of the chloroplast translocator on photosynthesis metabolism in transgenic potato plants. Planta 193:174–180
Heldt HW, Chon CJ, Maronde D, Herold A, Stankovic ZS, Walker DA, Kraminer A, Kirk MR, Heber U (1977) Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol 59:1146–1155
Herold A, Leegood RC, McNeil PH, Robinson SP (1981) Accumulation of maltose during photosynthesis in protoplasts isolated from spinach leaves treated with mannose. Plant Physiol 67:85–88
Kakefuda G, Duke SH (1989) Characterization of pea chloroplast D-enzyme (4-α-d-glucanotransferase. Plant Physiol 91:136–143
Kakefuda G, Duke SH, Hostak MS (1986) Chloroplast and extrachloroplastic starch-degrading enzymes in Pisum sativum L.. Planta 168:17–182
Kammerer B, Fischer K, Hilpert B, Schubert S, Gutensohn M, Weber A, Flügge U-I (1998) Molecular characterization of a carbon transporter in plastids from heterotrophic tissues: the glucose 6-phosphate phosphate antiporter. Plant Cell 10:105–117
Kofler H, Häusler RE, Schulz B, Gröner F, Flügge UI, Weber A (2000) Molecular characterisation of a new mutant allele of the plastidic phosphoglucomutase and complementation of the mutant with the wild-type cDNA. Mol Genet Genomics 263:978–986
Kruger NJ, ap Rees T (1983) Maltose metabolism by pea chloroplasts. Planta 158:179–184
Laby RJ, Kim D, Gibson SI (2001) The ram1 mutant of Arabidopsis exhibits severely decreased β-amylase activity. Plant Physiol 127:1798–1807
Lao NT, Schoneveld O, Mould RM, Hibberd JM, Gray JC, Kavanagh TA (1999) An Arabidopsis gene encoding a chloroplast-targeted β-amylase. Plant J 20:519–527
Leidreiter K, Heineke D, Heldt HW, Müller-Röber B (1995a) Leaf-specific antisense inhibition of starch biosynthesis in transgenic potato plants leads to an increase in photoassimilate export from source leaves during the light period. Plant Cell Physiol 36:615–624
Leidreiter K, Kruse A, Heineke D, Robinson DG, Heldt HW (1995b) Subcellular volumes and metabolite concentrations in potato (Solanum tuberosum cv désirée) leaves. Bot Acta 108:439–444
Levi C, Gibbs M (1976) Starch degradation in isolated chloroplasts. Plant Physiol 57:933–935
Levi C, Preiss J (1978) Amylopectin degradation in pea chloroplast extracts. Plant Physiol 61:218–220
Lowry OH, Passonneau JV (1972) A flexible system of enzymatic analysis. Academic Press, Orlando, pp 1–291
Lu Y, Sharkey TD (2003) The role of amylomaltase in maltose metabolism in the cytosol of photosynthetic cells. Planta DOI 10.1007/s00425-003-1127-z
Neuhaus HE, Schulte N (1996) Starch degradation in chloroplasts isolated from C3 or CAM (crassulacean acid metabolism)-induced Mesembryanthemum crystallinum L.. Biochem J 318:945–953
Peavey DG, Steup M, Gibbs M (1977) Characterization of starch breakdown in the intact spinach chloroplast. Plant Physiol 60:305–308
Riesmeier JW, Flügge U, Schulz B, Heineke D, Heldt HW, Willmitzer L, Frommer WB (1993) Antisense repression of the chloroplast triose phosphate translocator affects carbon partitioning in transgenic potato plants. Proc Natl Acad Sci USA 90:6160–6164
Ritte G, Raschke K (2003) Metabolite export of isolated guard cell chloroplasts of Vicia faba. New Phytol 159:195–202
Ritte G, Lloyd JR, Eckermann N, Rottmann A, Kossmann J, Steup M (2002) The starch-related R1 protein is an alpha-glucan, water dikinase. Proc Natl Acad Sci USA 99:7166–7171
Rost S, Frank C, Beck E (1996) The chloroplast envelope is permeable for maltose but not for maltodextrins. Biochim Biophys Acta 1291:221–227
Schäfer G, Heber U, Heldt HW (1977) Glucose transport into spinach chloroplasts. Plant Physiol 60:286–289
Scheidig A, Fröhlich A, Schulze S, Lloyd JR, Kossmann J (2002) Downregulation of a chloroplast-targeted β-amylase leads to a starch-excess phenotype in leaves. Plant J 30:581–591
Schleucher J, Vanderveer PJ, Sharkey TD (1998) Export of carbon from chloroplasts at night. Plant Physiol 118:1439–1445
Schneider A, Häusler RE, Kolukisaoglu Ü, Kunze R, van der Graaff E, Schwacke R, Catoni E, Desimone M, Flügge U-I (2002) An Arabidopsis thaliana knock-out mutant of the chloroplast triose phosphate/phosphate translocator is severely compromised only when starch synthesis, but not starch mobilisation is abolished. Plant J 32:685–699
Scott P, Kruger NJ (1994) Fructose-2,6-bisphospahte levels in mature leaves of tobacco (Nicotiana tabacum) and potato (Solanum tuberosum). Planta 193:16–20
Servaites JC, Geiger D (2002) Kinetic characteristics of chloroplast glucose transport. J Exp Bot 53:1–11
Servaites JC, Geiger DR, Tucci MA, Fondy B (1989) Leaf carbon metabolism and metabolite levels during a period of sinusoidal light. Plant Physiol 89:403–408
Sharkey TD, Vanerveer PJ (1989) Stromal phosphate concentration is low during feedback limited photosynthesis. Plant Physiol 91:679–684
Sharkey TD, Savitch LV, Vanderveer PJ, Micallef BJ (1992) Carbon partitioning in a Flaveria linearis mutant with reduced cytosolic fructose bisphosphatase. Plant Physiol 100:210–215
Shirokane Y, Ichikawa K, Suzuki M (2000) A novel enzymic determination of maltose. Carbohydr Res 329:699–702
Stitt M (1990) Fructose-2,6-bisphosphate in plants. Annu Rev Plant Physiol 41:153–185
Stitt M, ap Rees T (1979) Capacities of pea chloroplasts to catalyse the oxidative pentose phosphate pathway and glycolysis Phytochemistry 18:1905–1911
Stitt M, ap Rees T (1980) Carbohydrate breakdown by chloroplasts of Pisum sativum. Biochim Biophys Acta 627:131–143
Stitt M, Heldt HW (1981) Physiological rates of starch breakdown in isolated intact spinach chloroplasts. Plant Physiol 68: 755–761
Stitt M, Bulpin PV, ap Rees T (1978) Pathway of starch breakdown in photosynthetic tissues of Pisum sativum. Biochim Biophys Acta 544:200–214
Stitt M, Wirtz W, Heldt HW (1981) Metabolite levels during induction in the chloroplast and extrachloroplast compartments of spinach protoplasts. Biochim Biophys Acta 593:85–102
Stitt M, Wirtz W, Gerhardt R, Heldt HW, Spencer C, Walker D, Foyer C (1985) A comparative study of metabolite levels in plant leaf material in the dark. Planta 166:354–364
Sun ZT, Duke SH, Henson CA (1995) The role of pea chloroplast alpha-glucosidase in transitory starch degradation. Plant Physiol 108:211–217
Trethewey RN, ap Rees T (1994) The role of the hexose transporter in the chloroplasts of Arabidopsis thaliana L. Planta 195:168–174
Usuda H, Kalt-Torres W, Kerr PS, Huber SC (1987) Diurnal changes in maize leaf photosynthesis. II. Levels of metabolic intermediates of sucrose synthesis and the regulatory metabolite fructose 2,6-bisphosphate. Plant Physiol 83:289–293
Weber A, Servaites JC, Geiger DR, Kofler H, Hille D, Gröner F, Hebbeker U, Flügge U-I (2000) Identification, purification, and molecular cloning of a putative plastidic glucose translocator. Plant Cell 12:787–801
Wiese A, Gröner F, Sonnewald U, Deppner H, Lerchl J, Hebbeker U, Flügge U-I, Weber A (1999) Spinach hexokinase I is located in the outer envelope membrane of plastids. FEBS Lett 461:13–18
Winter H, Robinson DG, Heldt HW (1993) Subcellular volumes and metabolite concentrations in barley leaves. Planta 191:180–190
Winter H, Robinson DG, Heldt HW (1994) Subcellular volumes and metabolite concentrations in spinach leaves. Planta 193:530–535
Yu T-S, Lue W-L, Wang S-M, Chen J (2000) Mutation of Arabidopsis plastid phosphoglucose isomerase affects leaf starch synthesis and floral initiation. Plant Physiol 123:319–325
Yu T-S, Kofler H, Häusler RE, Hille D, Flügge U-I, Zeeman SC, Smith AM, Kossmann J, Lloyd J, Ritte G, Steup M, Lue W-L, Chen J, Weber A (2001) The Arabidopsis sex1 mutant is defective in the R1 protein, a general regulator of starch degradation In plants, and not in the chloroplast hexose transporter. Plant Cell 13:1907–1918
Zeeman SC, ap Rees T (1999) Changes in carbohydrate metabolism and assimilate export in starch-excess mutants of Arabidopsis. Plant Cell Environ 22:1445–1453
Zeeman SC, Northrop F, Smith AM, ap Rees T (1998) A starch-accumulating mutant of Arabidopsis thaliana deficient in a chloroplastic starch-hydrolysing enzyme Plant J 5:357–365
Zrenner R, Krause KP, Apel P, Sonnewald U (1996) Reduction of the cytosolic fructose-1,6-bisphosphatase in transgenic potato plants limits photosynthetic sucrose biosynthesis with no impact on plant growth and tuber yield. Plant J 9:671–681
Acknowledgements
This research was supported by the US Department of Energy under grant DE-FG02-99ER 20345. S.E.W. was supported in part by the Wisconsin Center for Space Automation and Robotics of UW-Madison. We thank Peter Vanderveer for help in starting this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weise, S.E., Weber, A.P.M. & Sharkey, T.D. Maltose is the major form of carbon exported from the chloroplast at night. Planta 218, 474–482 (2004). https://doi.org/10.1007/s00425-003-1128-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-1128-y