Abstract
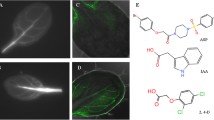
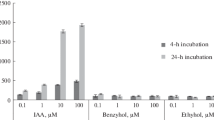
Agrobacterium tumefaciens-induced plant tumors accumulate considerable concentrations of free auxin. To determine possible mechanisms by which high auxin concentrations are maintained, we examined the pattern of auxin and flavonoid distribution in plant tumors. Tumors were induced in transformants of Trifolium repens (L.), containing the β-glucuronidase (GUS)-fused auxin-responsive promoter (GH3) or chalcone synthase (CHS2) genes, and in transformants of Arabidopsis thaliana (L.) Heynh., containing the GUS-fused synthetic auxin response element DR5. Expression of GH3::GUS and DR5::GUS was strong in proliferating metabolically active tumors, thus suggesting high free-auxin concentrations. Immunolocalization of total auxin with indole-3-acetic acid antibodies was consistent with GH3::GUS expression indicating the highest auxin concentration in the tumor periphery. By in situ staining with diphenylboric acid 2-aminoethyl ester, by thin-layer chromatography, reverse-phase high-performance liquid chromatography, and two-photon laser-scanning microscopy spectrometry, tumor-specific flavones, isoflavones and pterocarpans were detected, namely 7,4′-dihydroxyflavone (DHF), formononetin, and medicarpin. DHF was the dominant flavone in high free-auxin-accumulating stipules of Arabidopsis leaf primordia. Flavonoids were localized at the sites of strongest auxin-inducible CHS2::GUS expression in the tumor that was differentially modulated by auxin in the vascular tissue. CHS mRNA expression changes corresponded to the previously analyzed auxin concentration profile in tumors and roots of tumorized Ricinus plants. Application of DHF to stems, apically pretreated with α-naphthaleneacetic acid, inhibited GH3::GUS expression in a fashion similar to 1-N-naphthyl-phthalamic acid. Tumor, root and shoot growth was poor in inoculated tt4(85) flavonoid-deficient CHS mutants of Arabidopsis. It is concluded that CHS-dependent flavonoid aglycones are possibly endogenous regulators of the basipetal auxin flux, thereby leading to free-auxin accumulation in A. tumefaciens-induced tumors. This, in turn, triggers vigorous proliferation and vascularization of the tumor tissues and suppresses their further differentiation.









Similar content being viewed by others
Abbreviations
- CHS :
-
chalcone synthase
- CLSM:
-
confocal laser-scanning microscopy
- DHF:
-
7,4′-dihydroxyflavone
- DPBA:
-
diphenylboric acid 2-aminoethyl ester
- DR5 :
-
auxin response element
- GH3::GUS :
-
auxin-responsive promoter fused to β-glucuronidase
- IAA:
-
indole-3-acetic acid
- IAAM :
-
tryptophan monooxygenase
- IGS :
-
indole-3-glycerolphosphate synthase
- MTT:
-
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
- NIT :
-
nitrilase
- NAA:
-
α-naphthaleneacetic acid
- NPA:
-
1-N-naphthylphthalamic acid
- pi:
-
post infection
- 2PLSM:
-
two-photon laser-scanning microscopy
- RT–PCR:
-
reverse transcription–polymerase chain reaction
- tt4(85):
-
transparent testa: flavonoid-deficient mutant
References
Aloni R (1980) Role of auxin and sucrose in the differentiation of sieve and tracheary elements in plant tissue cultures. Planta 150:255–263
Aloni R (1995) The induction of vascular tissues by auxin and cytokinin. In: Davies PJ (ed) Plant hormones: physiology, biochemistry and molecular biology. Kluwer, Dordrecht, pp 531–546
Aloni R, Pradel KS, Ullrich CI (1995) The three-dimensional structure of vascular tissues in Agrobacterium tumefaciens-induced crown galls and in the host stems of Ricinus communis L. Planta 196:597–605
Aloni R, Wolf A, Feigenbaum P, Avni A, Klee HJ (1998) The Never ripe mutant provides evidence that tumor-induced ethylene controls the morphogenesis of Agrobacterium tumefaciens-induced crown galls on tomato stems. Plant Physiol 117:841–849
Aloni R, Schwalm K, Langhans M, Ullrich CI (2003) Gradual shifts in sites of free auxin-production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta 216:841–853
Arioli T, Howels PA, Weinman JJ, Rolfe BG (1994) In Trifolium subterraneum, chalcone synthase is encoded by a multigene family. Gene 138:79–86
Bernasconi P, Patel BC, Reagan JD, Subramanian MV (1996) The N-1-naphthylphthalamic acid-binding protein is an integral membrane protein. Plant Physiol 111:427–433
Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK (2001) Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126:524–535
Djordjevic MA, Mathesius U, Arioli T, Weinman JJ, Gärtner E (1997) Chalcone synthase gene expression in transgenic subterranean clover correlates with localised accumulation of flavonoids. Aust J Plant Physiol 24:119–132
Friml J, Benková E, Blilou I, Wisniewska J, Hamann T, Ljung K, Woody S, Sandberg G, Scheres B, Jürgens G, Palme K (2002b) AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 108:661–673
Gális I, Šimek P, Van Onckelen HA, Kakiuchi Y, Wabiko H (2002) Resistance of transgenic tobacco seedlings expressing the Agrobacterium tumefaciens C58-6b gene, to growth-inhibitory levels of cytokinin is associated with elevated IAA levels and activation of phenylpropanoid metabolism. Plant Cell Physiol 43:939–950
Gehrig HH, Schüßler A, Kluge M (1996) Geosiphon pyriforme, a fungus forming endocytobiosis with Nostoc (Cyanobacteria), is an ancestral member of the Glomales: evidence by SSU rRNA analysis. J Mol Evol 43:71–81
Geldner N, Friml J, Stierhof Y-D, Jürgens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413:425–428
Hagen G, Martin G, Li Y, Guilfoyle TJ (1991) Auxin-induced expression of the soybean GH3 promoter in transgenic tobacco plants. Plant Mol Biol 17:567–579
Harborne JB, Baxter H (1999) The handbook of natural flavonoids, vol 2. Wiley, Chichester
Heller W, Forkmann G (1993) Biosynthesis of flavonoids. In: Harborne JB (ed) The flavonoids: advances in research since 1986. Chapman and Hall, London, pp 499–535
Hirsch AM (1992) Developmental biology of legume nodulation. New Phytol 122:211–237
Howles PA, Arioli T, Weinman JJ (1995) Nucleotide sequence of additional members of the gene family encoding chalcone synthase in Trifolium subterraneum. Plant Physiol 107:1035–1036
Humphreys JM, Chapple C (2000) Molecular ‘pharming’ with plant P450 s. Trends Plant Sci 5:271–272
Hutangura P, Mathesius U, Jones MGK, Rolfe BG (1999) Auxin induction is a trigger for root gall formation caused by root-knot nematodes in white clover and is associated with the activation of the flavonoid pathway. Aust J Plant Physiol 26:221–231
Jacobs M, Gilbert SF (1983) Basal localization of the presumptive auxin transport carrier in pea stem cells. Science 220:1297–1300
Jacobs M, Rubery PH (1988) Naturally occurring auxin transport regulators. Science 241:346–349
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5:387–405
Klee H, Montoya A, Horodyski F, Lichtenstein C, Garfinkel D, Fuller S, Flores C, Peschon J, Nester E, Gordon M (1984) Nucleotide sequence of the tms genes of the pTiA6NC octopine Ti plasmid: two gene products involved in plant tumorigenesis. Proc Natl Acad Sci USA 81:1728–1732
Langhans M, Ratajczak R, Lützelschwab M, Michalke W, Wächter R, Fischer-Schliebs E, Ullrich CI (2001) Immunolocalization of plasma-membrane H+-ATPase and tonoplast-type pyrophosphatase in the plasma membrane of the sieve element–companion cell complex in the stem of Ricinus communis L. Planta 213:11–19
Larkin PJ, Gibson JM, Mathesius U, Weinmann JJ, Gärtner E, Hall E, Tanner GJ, Rolfe BG, Djordjevic MA (1996) Transgenic white clover. Studies with the auxin-responsive promoter, GH3, in root gravitropism and lateral root development. Transgenic Res 5:325–335
Lawson CG, Djordjevic MA, Weinmann JJ, Rolfe BG (1994) Rhizobium inoculation and physical wounding result in the rapid induction of the same chalcone synthase copy in Trifolium subterraneum. Mol Plant Microbe Interact 7:498–507
Logemann J, Schell J, Willmitzer L (1987) Improved method for the isolation of RNA from plant tissues. Anal Biochem 16:16–20
Löw R, Rausch T (1994) Sensitive non-radioactive Northern blots using alkaline transfer of total RNA and PCR-amplified biotinylated probes. Biotechnology 17:1026–1030
Marchant A, Bhalerao R, Casimiro I, Eklöf J, Casero PJ, Bennett M, Sandberg G (2002) AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14:589–597
Mathesius U (2001) Flavonoids induced in cells undergoing nodule organogenesis in white clover are regulators of auxin breakdown by peroxidase. J Exp Bot 52:419–426
Mathesius U, Bayliss C, Weinman JJ, Schlaman HRM, Spaink HP, Rolfe BG, McCully ME, Djordjevic MA (1998) Flavonoids synthesized in cortical cells during nodule initiation are early developmental markers in white clover. Mol Plant Microbe Interact 11:1223–1232
Müller A, Düchting P, Weiler, EW (2002) A multiplex GC–MS/MS technique for the sensitive and qualitative single-run analysis of acidic phytohormones and related compounds, and its application to Arabidopsis thaliana. Planta 216:44–56
Murphy A, Peer WA, Taiz L (2000) Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 211:315–324
Palme K, Gälweiler L (1999) PIN-pointing the molecular basis of auxin transport. Curr Opin Plant Biol 2:375–381
Popov N, Schmitt M, Schulzek S, Matthies H (1975) Eine störungsfreie Mikromethode zur Bestimmung des Proteingehaltes in Gewebehomogenaten. Acta Biol Med Ger 34:1441–1446
Rezmer C, Schlichting R, Wächter R, Ullrich, CI (1999) Identification and localization of transformed cells in Agrobacterium tumefaciens-induced plant tumors. Planta 209:399–405
Ryder TB, Hedrick SA, Bell JN, Liang X, Clouse SD, Lamb CJ (1987) Organization and differential activation of a gene family encoding the plant defense enzyme chalcone synthase in Phaseolus vulgaris. Mol Gen Genet 210:219–233
Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle TJ, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, Scheres B (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99:463–472
Saslowsky DE, Dana CD, Winkel-Shirley B (2000) An allelic series for the chalcone synthase locus in Arabidopsis. Gene 255:127–138
Sitbon F, Sundberg B. Olsson O, Sandberg G (1991) Free and conjugated indoleacetic acid (IAA) contents in transgenic tobacco plants expressing the iaaM and iaaH IAA biosynthesis gene from Agrobacterium tumefaciens. Plant Physiol 95:480–485
Stenlid G (1976) Effects of flavonoids on the polar transport of auxins. Physiol Plant 38:262–266
Suttle JC (1988) Effect of ethylene treatment on polar IAA-transport, net IAA uptake and specific binding of N-1-naphthylphthalamic acid in tissues and microsomes isolated from etiolated pea epicotyls. Plant Physiol 88:795–799
Turunen M, Heller W, Stich S, Sandermann H, Sutinen M-L, Norokopi Y (1999) The effects of UV exclusion on the soluble phenolics of young Scots pine seedlings in the subarctic. Environ Pollut 106:219–228
Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9:1963–1971
Veselov SY, Kudoyarova GR, Egutkin NL, Gyuli-Zade VZ, Mustafina AR, Kof EM (1992) Modified solvent partitioning scheme providing increased specificity and rapidity of immunoassay for indole-3-acetic acid. Physiol Plant 86:93–96
Veselov D, Langhans M, Hartung W, Aloni R, Feussner I, Götz C, Veselova S, Schlomski S, Dickler C, Bächmann K, Ullrich CI (2003) Development of Agrobacterium tumefaciens C58-induced plant tumors and impact on host shoots are controlled by a cascade of jasmonic acid, auxin, cytokinin, ethylene and abscisic acid. Planta 216:512–522
Wächter R, Fischer K, Gäbler R, Kühnemann F, Urban W, Bögemann GM, Voesenek LAC J, Blom CWPM, Ullrich CI (1999) Ethylene production and ACC-accumulation in Agrobacterium tumefaciens-induced plant tumors and their impact on tumor and host stem structure and function. Plant Cell Environ 22:1263–1273
Weiler EW, Spanier K (1981) Phytohormones in the formation of crown gall tumors. Planta 153:326–337
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) to C.I.U.; M.L. was supported by a Hessen Ph.D. fellowship. We thank Dr. Ulrike Mathesius (Genomic Interactions Group, Research School of Biological Sciences, National University, Canberra, Australia) for the kind gift of transformed white clover plants and stimulating discussions, and Dr. Rosalia Deeken (Würzburg University, Germany) for her spontaneous supply of Arabidopsis (Ler) plants.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of Professor Wilhelm Simonis (1909–2003)
Rights and permissions
About this article
Cite this article
Schwalm, K., Aloni, R., Langhans, M. et al. Flavonoid-related regulation of auxin accumulation in Agrobacterium tumefaciens-induced plant tumors. Planta 218, 163–178 (2003). https://doi.org/10.1007/s00425-003-1104-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-1104-6