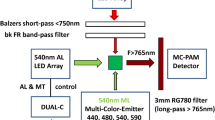
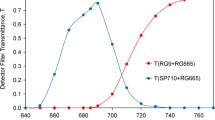
Abstract
Light-induced fluorescence changes (LIFCs) were detected in sporangiophores of the blue-light-sensitive fungus Phycomyces blakesleeanus (Burgeff). The LIFCs can be utilized as a spectrophotometric assay for blue-light photoreceptors and for the in vivo characterization of their photochemical primary reactions. Blue-light irradiation of sporangiophores elicited a transient decrease and subsequent regeneration of flavin-like fluorescence emission at 525 nm. The signals recovered in darkness in about 120 min. In contrast to blue light, near-UV (370 nm) caused an increase in the fluorescence emission at 525 nm. Because the LIFCs were altered in a light-insensitive madC mutant with a defective photoreceptor, the fluorescence changes must be associated with early photochemical events of the transduction chain. Action spectra for the fluorescence changes at 525 nm showed major peaks near 470 and 600 nm. Double-pulse experiments involving two consecutive pulses of either blue and near-UV, blue and red, or near-UV and red showed that the responses depended on the sequence in which the different wavelengths were applied. The results indicate a blue-light receptor with intermediates in the near-UV, blue and red spectral regions. We explain the results in the framework of a general model, in which the three redox states of the flavin photoreceptor, the oxidized flavin (Fl), the flavo-semiquinone (FlH·), and the flavo-hydroquinone (FlH2) are each acting as chromophores with their own characteristic photochemical primary reactions. These consist of the photoreduction of the oxidized flavin generating semiquinone, the photoreduction of the semiquinone generating hydroquinone, and the photooxidation of the flavo-hydroquinone regenerating the pool of oxidized flavins. The proposed mechanism represents a photocycle in which two antagonistic photoreceptor forms, Fl and FlH2, determine the pool size of the biological effector molecule, the flavo-semiquinone. The redox changes that are associated with the photocycle are maintained by redox partners, pterins, that function in the near-UV as secondary chromophores.















Similar content being viewed by others
Abbreviations
- FAD:
-
flavin adenine dinucleotide
- Fl:
-
oxidized flavin
- FlH:
-
flavo-semiquinone radical
- FlH2 :
-
flavo-hydroquinone
- LIAC:
-
light-induced absorbance change
- LIFC:
-
light-induced fluorescence change
- Pt:
-
oxidized pterin
- PtH2 :
-
dihydro-pterin
- PtH4 :
-
tetrahydro-pterin
References
Ahmad M, Cashmore AR (1993) HY4 gene of Arabidopsis thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366:162–166
Ahmad M, Cashmore AR (1996) Seeing blue: the discovery of cryptochrome. Plant Mol Biol 30:851–861
Ahmad M, Heil M, Black RC, Giovani B, Galland P, Lardemer D (2002) Action spectrum for hypocotyl growth inhibition suggests dosage-dependent synergism among cryptochrome photoreceptors of Arabidopsis thaliana. Plant Physiol 129:774–785
Bergman K, Eslava AP, Cerdá-Olmedo E (1973) Mutants of Phycomyces with abnormal phototropism. Mol Gen Genet 123:1–16
Berns DS, Vaughn JR (1970) Studies of the photopigment system in Phycomyces. Biochem Biophys Res Commun 39:1094–1103
Brodhun B, Häder D-P (1990) Photoreceptor proteins and pigments in the paraflagellar body of the flagellate Euglena gracilis. Photochem Photoiol 58:270–274
Brodhun B, Häder D-P (1993) UV-induced damage of photoreceptor proteins in the paraflagellar body of Euglena gracilis. Photochem Photobiol 58:270–274
Butler WL, Norris KH, Siegelman HW, Hendricks SB (1959) Detection, assay, and preliminary purification of the pigment controlling photoresponsive development of plants. Proc Natl Acad Sci USA 45:1703–1708
Chen XY, Xiong YQ, Lipson ED (1993) Action spectrum for subliminal light control of adaptation in Phycomyces phototropism. Photochem Photobiol 58:425–431
Christie JM, Reymond P, Powell GK, Bernasconi P, Raibekas A, Liscum E, Briggs WR (1998) Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science 282:1698–1701
Christie JM, Salomon M, Nozue K, Wada M, Briggs WR (1999) LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites for the chromophore flavin mononucleotide. Proc Natl Acad Sci USA 96:8779–8783
Delbrück M, Shropshire W Jr (1960) Action and transmission spectra of Phycomyces. Plant Physiol 35:194–204
Delbrück M, Katzir A, Presti D (1976) Responses of Phycomyces indicating optical excitation of the lowest triplet state of riboflavin. Proc Natl Acad Sci USA 73:1969–1973
Ehrenberg A, Hemmerich P (1968) Flavoenzymes: chemistry and molecular biology. In: Singer TP (ed) Biological oxidations. Interscience Publishers, New York, pp 239–262
Fries V, Krockert T, Grolig F, Galland P (2002) Statoliths in Phycomyces: spectrofluorometric characterization of octahedral protein crystals. J Plant Physiol 159:39–47
Galland P (1983) Action spectra of photogeotropic equilibrium in Phycomyces wild type and three behavioural mutants. Photochem Photobiol 37:221–228
Galland P (1998) Reception of far-ultraviolet light in Phycomyces: antagonistic interaction with blue and red light. Planta 205:269–276
Galland P, Lipson ED (1985) Modified action spectra of photogeotropic equilibrium in Phycomyces blakesleeanus with defects in genes madA, madB, madC, and madH. Photochem Photobiol 41:331–335
Galland P, Russo VEA (1984) Light and dark adaptation in Phycomyces phototropism. J Gen Physiol 84:101–118
Galland P, Senger H (1988) The role of pterins in the photoreception and metabolism of plants. Photochem Photobiol 48:811–820
Galland P, Senger H (1991) Flavins as possible blue-light photoreceptors. In: Holmes GH (ed) Photoreceptor evolution and function. Academic Press, London, pp 65–124
Galland P, Pandya A, Lipson ED (1984) Wavelength dependence of dark adaptation in Phycomyces phototropism. J Gen Physiol 84:739–751
Galland P, Orejas M, Lipson ED (1989) Light-controlled adaptation kinetics in Phycomyces: evidence for a novel yellow-light absorbing pigment. Photochem Photobiol 49:493–500
Galland P, Keiner P, Dörnemann D, Senger H, Brodhun B, Häder D-P (1990) Pterin- and flavin-like fluorescence associated with isolated flagella of Euglena gracilis. Photochem Photobiol 51:675–680
Galland P, Amon S, Senger H, Russo VEA (1995) Blue light reception in Phycomyces: red light sensitization in madC mutants. Bot Acta 108:344–350
Hohl N, Galland P, Senger H (1992a) Altered pterin patterns in photobehavioral mutants of Phycomyces blakesleeanus. Photochem Photobiol 55:239–246
Hohl N, Galland P, Senger H (1992) Altered flavin patterns in photobehavioral mutants of Phycomyces blakesleeanus. Photochem Photobiol 55:247–255
Iseki M, Matsunaga S, Muraka A, Ohno K, Shiga K, Yoshida K, Sugai M, Takahashi T, Hori T, Watanabe M (2002) A blue-light-activated adenylyl cyclase mediates photoavoidance in Euglena gracilis. Nature 415:1047–1051
Klemm E, Ninnemann H (1978) Correlation between absorbance change and a physiological response induced by blue light in Neurospora. Photochem Photobiol 28:227–230
Leong T-Y, Vierstra RD, Briggs WR (1981) A blue light-sensitive cytochrome–flavin complex from corn coleoptiles — further characterization. Photochem Photobiol 34:697–703
Lin C (2000) Plant blue-light receptors. Trends Plant Sci 5:337-342
Lin C, Robertson DE, Ahmad M, Raibekas AA, Jorns MS, Dutton PL, Cashmore AR (1995) Association of flavin adenine dinucleotide with the Arabidopsis blue-light receptor CRY1. Science 269:968–970
Lipson ED, Presti D (1977) Light-induced absorbance changes in Phycomyces photomutants. Photochem Photobiol 25:203–208
Löser G, Schäfer E (1986) Are there several photoreceptors involved in phototropism of Phycomyces blakesleeanus? Kinetic studies of dichromatic irradiation. Photochem Photobiol 43:195–204
Malhotra K, Kim ST, Batschauer A, Dawut L, Sancar A (1995) Putative blue-light photoreceptors from Arabidopsis thaliana and Sinapis alba with a high-degree of sequence homology to DNA photolyase contain the 2 photolyase cofactors but lack DNA repair activity. Biochemistry 34:6892–6899
Ootaki T, Lighty AC, Delbrück M, Hsu, W-J (1973) Complementation between mutants of Phycomyces deficient with respect to carotenogenesis. Mol Gen Genet 121:57–70
Palmer G, Massey V (1968) Mechanisms of flavoprotein catalysis. In: Singer TP (ed) Biological oxidations. Interscience Publishers, New York, pp 263–300
Poff KI, Butler WL (1974) Absorbance changes induced by blue light in Phycomyces blakesleeanus and Dictyostelium discoideum. Nature 248:799–801
Presti D, Hsu W-J, Delbrück M (1977) Phototropism in Phycomyces mutants lacking β-carotene. Photochem Photobiol 26:403–405
Sancar A (1994) Structure and function of DNA photolyase. Biochemistry 33:2–9
Schimek C, Eibel P, Grolig F, Horie T, Ootaki T, Galland P (1999) Gravitropism in Phycomyces: a role for sedimenting protein crystals and floating lipid globules. Planta 210:132–142
Schmidt W (1980) Physiological bluelight reception. Struct Bonding Berlin 41:1–44
Schmidt W (1995) Novel single-beam optical spectrophotometer for fast luminescence, absorption, and reflection measurements of turbid materials. Opt Eng 34:589–595
Schmidt W (1997) A multipurpose, fast scan spectrophotometer for measuring turbid (biological) materials. Springer. Experimental Biology Online (EB): http://science.springer.de/ebo/abstract/1997/sla97_3.htm
Schmidt W, Galland P (1999) Light-induced absorbance changes in Phycomyces: evidence for cryptochrome-associated flavosemiquinones. Planta 208:274–282
Schmidt W, Thomson K, Butler WL (1977) Cytochrome b in plasma membrane enriched fractions from several photoresponsive organisms. Photochem Photobiol 26:407–411
Schmidt W, Galland P, Senger H, Furuya M (1990) Microspectrophotometry of Euglena gracilis. Pterin- and flavin-like fluorescence in the paraflagellar body. Planta 182:375–381
Sutter RP (1975) Mutations affecting sexual development in Phycomyces blakesleeanus. Proc Natl Acad Sci USA 72:127–130
Trad CH, Lipson ED (1987) Biphasic fluence–response curves and derived action spectra for light-induced absorbance changes in Phycomyces blakesleeanus. J Photochem Photobiol B Biol 1:169-180
Trad CH, Horwitz BA, Lipson ED (1988) Light-induced absorbance changes in extracts of Phycomyces sporangiophores: modifications in night-blind mutants. J Photochem Photobiol B Biol 1:305–314
Acknowledgements
The excellent technical assistance by Sigrid Völk, Marko Göttig and Agnes Debelius is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galland, P., Tölle, N. Light-induced fluorescence changes in Phycomyces: evidence for blue light-receptor associated flavo-semiquinones. Planta 217, 971–982 (2003). https://doi.org/10.1007/s00425-003-1068-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-1068-6