Abstract
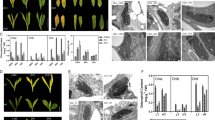
Cinnamoyl CoA reductase (CCR; EC 1.2.1.44) is the first enzyme specific to the biosynthetic pathway leading to monolignols. Arabidopsis thaliana (L.) Heynh. plants transformed with a vector containing a full-length AtCCR1 cDNA in an antisense orientation were obtained and characterized. The most severely down-regulated homozygous plants showed drastic alterations to their phenotypical features. These plants had a 50% decrease in lignin content accompanied by changes in lignin composition and structure, with incorporation of ferulic acid into the cell wall. Microscopic analyses coupled with immunolabelling revealed a decrease in lignin deposition in normally lignified tissues and a dramatic loosening of the secondary cell wall of interfascicular fibers and vessels. Evaluation of in vitro digestibility demonstrated an increase in the enzymatic degradability of these transgenic lines. In addition, culture conditions were shown to play a substantial role in lignin level and structure in the wild type and in the effects of AtCCR1 repression efficiency.





Similar content being viewed by others

Abbreviations
- ASCCR:
-
CCR anti-sense plants
- CC:
-
culture conditions
- CCR:
-
cinnamoyl CoA reductase
- G:
-
guaiacyl units
- H:
-
hydroxyphenyl units
- IVDMD:
-
in vitro dry matter digestibility
- IVNDFD:
-
in vitro NDF digestibility
- NDF:
-
neutral detergent fiber
- S:
-
syringyl units
- TEM:
-
transmission electron microscopy
- WS:
-
Wassilevskija
References
Aufrère J, Michalet-Doreau B (1983) In vivo digestibility and prediction of digestibility of some by-products. European Economic Community seminar, 26–29 September 1983. Mlle Gontrode, Belgium
Baucher M, Monties B, Van Montagu M, Boerjan W (1998) Biosynthesis and genetic engineering of lignin. Crit Rev Plant Sci 17:125–197
Bechtold N, Ellis J, Pelletier G (1993) In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. CR Acad Sci Paris, Sci Vie 316:1194–1199
Becker D (1990) Binary vectors which allow the exchange of plant selectable markers and reporter genes. Nucleic Acids Res 18:203
Boudet AM (2000) Lignins and lignification: selected issues. Plant Physiol Biochem 38:81–96
Burlat V, Kwon M, Davin LB, Lewis NG. (2001). Dirigent proteins and dirigent sites in lignifying tissues. Phytochemistry 57:883–897
Chabannes M, Ruel K, Yoshinaga A, Chabbert B, Jauneau A, Joseleau JP, Boudet AM (2001a) In situ analysis of lignins in transgenic tobacco reveals a differential impact of individual transformations on the spatial patterns of lignin deposition at the cellular and subcellular levels. Plant J 28:271–282
Chabannes M, Barakate A, Lapierre C, Marita JM, Ralph J, Pean M, Danoun S, Halpin C, Grima-Pettenati J, Boudet AM (2001b) Strong decrease in lignin content without significant alteration of plant development is induced by simultaneous down-regulation of cinnamoyl CoA reductase (CCR) and cinnamyl alcohol dehydrogenase (CAD) in tobacco plants. Plant J 28:257–270
Chapple CCS, Vogt T, Ellis BE, Somerville CR (1992) An Arabidopsis mutant defective in the general phenylpropanoid pathway. Plant Cell 4:1413–1424
Dence C (1992) Lignin determination. In: Dence C, Lin S (eds) Methods in lignin chemistry. Springer, Berlin Heidelberg New York, pp 33–61
Dharmawardhana DP, Ellis BE, Carlson JE (1992) Characterization of vascular lignification in Arabidopsis thaliana. Can J Bot 70:2238–2244
Dolstra O, Medema JH (1990) An effective screening method for genetic improvement of cell-wall digestibility in forage maize. In: Proceedings of the 15th congress maize and sorghum section of Eucarpia, Baden, Austria, June 4–8, pp 258–270
Estelle M, Somerville CR (1987) Auxin-resistant mutants of Arabidopsis thaliana with an altered morphology. Mol Gen Genet 206:200–206
Goering HK, Van Soest PJ (1970) Forage fiber analysis (apparatus, reagents, procedures, and some applications). USDA ARS Agricultural handbook 379. US government Printing Office, Washington, DC
Humphreys JM, Chapple C (2002) Rewriting the lignin roadmap. Curr Opin Plant Biol 5:224–229
Humphreys JM, Hemm MR, Chapple C (1999) New routes for lignin biosynthesis defined by biochemical characterization of recombinant ferulate 5-hydroxylase, a multifunctional cytochrome P450-dependent monooxygenase. Proc Natl Acad Sci USA 96:10045–10050
Jones L, Ennos AR, Turner SR (2001) Cloning and characterization of irregular xylem4 (irx4): a severely lignin-deficient mutant of Arabidopsis. Plant J 26:205–216
Joseleau J-P, Ruel K (1997) Study of lignification by noninvasive techniques in growing maize internodes—an investigation by Fourier transform infrared, cross-polarisation-magic angle spinning 13C-nuclear magnetic resonance spectroscopy and immunocytochemical transmission electron microscopy. Plant Physiol 114:1123–1133
Koncz C, Schell J (1986) The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204:383–396
Lacombe E, Hawkins S, Van Doorselaere J, Piquemal J, Goffner D, Poeydomenge O, Boudet AM, Grima-Pettenati J (1997) Cinnamoyl CoA reductase, the first committed enzyme of the lignin branch biosynthetic pathway: cloning, expression and phylogenetic relationships. Plant J 11:429–441
Lapierre C, Pollet B, Rolando R (1995) New insights into the molecular architecture of hardwood lignins by chemical degradation methods. Res Chem Intermed 21:397–412
Lauvergeat V, Lacomme C, Lacombe E, Lasserre E, Roby D, Grima-Pettenati J (2001) Two cinnamoyl-CoA reductase (CCR) genes from Arabidopsis thaliana are differentially expressed during development and in response to infection with pathogenic bacteria. Phytochemistry 57:1187–1195
Lewis NG, Davin LB (1994) Evolution of lignan and neolignan biochemical pathways. In: Nes WD (ed) Isopentonoids and other natural products: evolution and function. ACS Symposium Series, No 562, Washington DC, pp 202–246
Li L, Popko JL, Umezawa T, Chiang VL (2000) 5-hydroxyconiferyl aldehyde modulates enzymatic methylation for syringyl monolignol formation, a new view of monolignol biosynthesis in angiosperms. J Biol Chem 275:6537–6545
Li L, Cheng XF, Leshkevich J, Umezawa T, Harding SA, Chiang VL (2001) The last step of syringyl monolignol biosynthesis in angiosperms is regulated by a novel gene encoding sinapyl alcohol dehydrogenase. Plant Cell 13:1567–1585
Méchin V, Argillier O, Barrière Y, Mila I, Polet B, Lapierre C (2000). Relationships of cell-wall composition to in vitro cell-wall digestibility of maize inbred line stems. J Sci Food Agric 80:574–580
Meyer K, Shirley AM, Cusumano JC, Bell-Lelong DA, Chapple CCS (1998) Lignin monomer composition is determined by the expression of a cytochrome P450-dependent monooxygenase in Arabidopsis. Proc Natl Acad Sci USA 95:6619–6623
Osakabe K, Tsao CC, Li L, Popko JL, Umezawa T, Carraway DT, Smeltzer RH, Joshi CP, Chiang VL (1999) Coniferyl aldehyde 5-hydroxylation and methylation direct syringyl lignin biosynthesis in angiosperms. Proc Natl Acad Sci USA 96:8955–8960
Pinçon G, Chabannes M, Lapierre C, Pollet B, Ruel K, Joseleau JP, Boudet AM, Legrand M (2001) Simultaneous down-regulation of caffeic/5-hydroxy ferulic acid-O-methyltransferase I and cinnamoyl-coenzyme A reductase in the progeny from a cross between tobacco lines homozygous for each transgene. Consequences for plant development and lignin synthesis. Plant Physiol 126:145–155
Piquemal J, Lapierre C, Myton K, O'Connell A, Schuch W, Grima-Pettenati J, Boudet A-M (1998) Down-regulation in cinnamoyl-CoA reductase induces significant changes of lignins profiles in transgenic tobacco plants. Plant J 13:71–83
Ruel K, Barnoud F, Eriksson KE (1981) Micromorphological and ultrastructural aspects of spruce wood degradation by wild type Sporotrichum pulverulentum and its cellulase-less mutant Cel 44. Holzforschung 35:157–171
Ruel K, Faix O, Joseleau JP (1994) New immunogold probes for studying the distribution of the different lignin types during plant cell wall biogenesis. J Trace Microprobe Tech 12:247–265
Ruel K, Burlat V, Joseleau JP (1999) Relationship between ultrastructural topochemistry of lignin and wood properties. Int Assoc Wood Anat J 20:203–211
Ruel K, Chabannes M, Boudet A-M, Legrand M, Joseleau J-P (2001) Reassessment of qualitative changes in lignification of transgenic tobacco plants and their impact on cell wall assembly. Phytochemistry 57:875–882
Ruel K, Montiel MD, Goujon T, Jouanin L, Burlat V, Joseleau JP (2002) Inter-relation between lignin deposition and polysaccharide matrices during the assembly of the plant cell walls. Plant Biol 3:1–7
Sarkanen KV, Hergert HL (1971) Classification and distribution. In: Sarkanen KV, Ludwig CH (eds) Lignins: occurrence, formation, structure and reactions. Wiley-Interscience, New York, pp 43–94
Srebotnik E, Messner K (1994) A simple method that uses differential staining and light microscopy to assess the selectivity of wood delignification by white rot fungi. Appl Environ Microbiol 60:1383–1386
Struik PC (1983) Physiology of forage maize (Zea mays L.) in relation to its productivity. PhD thesis, Wageningen, The Netherlands
Terashima N, Fukushima K, Takabe K (1993) Comprehensive model of the lignified plant cell wall. In: Jung HG, Buxton DR, Hatfield RD, Ralph J (eds) Forage cell wall structure and digestibility. ASA Madison, Wis, pp 247–270
Thiery JP (1967) Mise en évidence des polysaccharides sur coupes fines en microscopie électronique. J Microsc 6:987–1017
Turner SR, Somerville CR (1997) Collapsed xylem phenotype of Arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall. Plant Cell 9:689–701
Verwoerd TC, Dekker BMM, Hoekema A (1989) A small-scale procedure for the rapid isolation of plant RNAs. Nucl Acids Res 17:2362
Zhong R, Morrison WH, Negrel J, Ye ZH (1998) Dual methylation pathways in lignin biosynthesis. Plant Cell 10:2033–2045
Zhong R, Ripperger. A, Ye ZH (2000). Ectopic deposition of lignin in the pith of stems of two Arabidopsis mutants. Plant Physiol 123:59–70
Acknowledgements
The authors are very grateful to Magalie Pichon (CNRS, Toulouse) who performed the CCR activity assays and to Frédéric Legée (INRA-INA, Grignon) who did the Klason measurements. They thank Jean-Pascal Meunier, Joël Talbotec and Hervé Ferry (INRA, Versailles) who took care of the plants in the greenhouse and the climatized chambers. The authors also wish to thank Deborah Goffner (CNRS, Toulouse) for corrections and critical review of the manuscript. Cell wall digestibility assays were performed in the framework of the Génoplante program Af 1999-011.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goujon, T., Ferret, V., Mila, I. et al. Down-regulation of the AtCCR1 gene in Arabidopsis thaliana: effects on phenotype, lignins and cell wall degradability. Planta 217, 218–228 (2003). https://doi.org/10.1007/s00425-003-0987-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-0987-6